Advanced Biological Releases First Commercial CE-IVD Registered COVID-19 rRT-PCR Assay Also Validated for Saliva
By LabMedica International staff writers
Posted on 08 Jul 2020
Advanced Biological Laboratories (ABL Luxembourg) has announced the CE-IVD marking of its UltraGene Combo2Screen SARS-CoV-2 qPCR assay. Posted on 08 Jul 2020
The UltraGene Combo2Screen SARS-CoV-2 qPCR assay is the first qPCR detection kit now validated with SARS-CoV-2 RNA extracted from nasopharyngeal swabs and saliva. The collection of saliva samples is not invasive and allows easier and broader population testing and screening. This optimization consisting in using saliva specimen as an input material for the SARS CoV-2 RNA extraction and qPCR detection, rather than traditional nasopharyngeal swabs, helps in improving the workflow, costs and convenience of the test together with the comfort of the patients. It allows targeting of asymptomatic patients, increasing acceptance of patients for testing and reducing workload and risk of infection for healthcare professionals performing nasopharyngeal or oropharyngeal collections. Patients collect saliva samples under the supervision of healthcare professionals under a simpler and painless collection protocol.
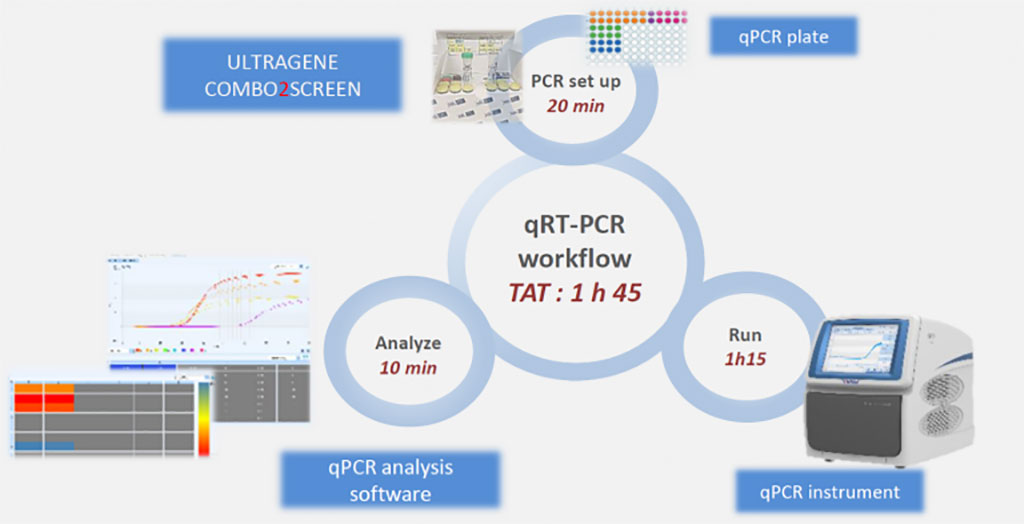
Image: qRT-PCR Workflow (Photo courtesy of Advanced Biological Laboratories)
The UltraGene Combo2Screen CE-IVD assay targets two highly conserved regions of the SARS-CoV-2 genome (Nucleocapsid (N) and Envelop (E) genes) in a multiplex format which includes an internal control. The test remains one of the most sensitive and easy to use assays from the market (~200 cp/mL), either with naso-oropharyngeal or saliva samples. It is compatible with most of the automated or manual RNA extraction methods, as well as with most of the qPCR instruments.
“We are proud to enhance our COVID-19 line of solutions. Converting the assay into a non-invasive fluid saliva collection workflow should significantly help increasing the number of people being tested and ease the access to diagnostics to fragile population such as children or elderly patients and to asymptomatic patients” said Dr. Chalom Sayada, CEO of ABL. “Proactive saliva collection strategy at schools and business places shall be foreseen to monitor prospectively SARS-CoV-2 infection”
Related Links:
Advanced Biological Laboratories














