New ELISA-Based COVID-19 Test Detects and Ranks SARS-CoV-2 Neutralizing Antibodies by Their Potency
A new enzyme-linked immunosorbent assay (ELISA)-based COVID-19 diagnostic test detects and ranks SARS-CoV-2 neutralizing antibodies by their potency in a simple and relatively short test time. More...04 Dec 2020
Using Combination of Several SARS-CoV-2 Antibody Tests May Give Best Results for Detecting COVID-19, Finds Study
A new study indicates that the sensitivity of tests used to detect SARS-CoV-2 antibodies in a blood sample may differ significantly and a combination of several tests may give the best result in detecting COVID-19. More...04 Dec 2020


Global Swab and Viral Transport Medium Market to Reach USD 2.2 Billion by 2030 on Back of COVID-19 Pandemic
The global swab and viral transport medium (VTM) market was valued at USD 0.9 billion in 2019 and will witness a moderate growth rate of ~3% from 2020 to 2030 to reach USD 2.2 billion by 2030, driven by the onslaught of COVID-19 and increasing R&D activities. More...03 Dec 2020
Virus-Like Probes Could Help Make Rapid COVID-19 Testing More Accurate and Reliable
A team of nanoengineers has developed new and improved probes, known as positive controls, that could make it easier to validate rapid, point-of-care diagnostic tests for COVID-19 across the globe. More...02 Dec 2020
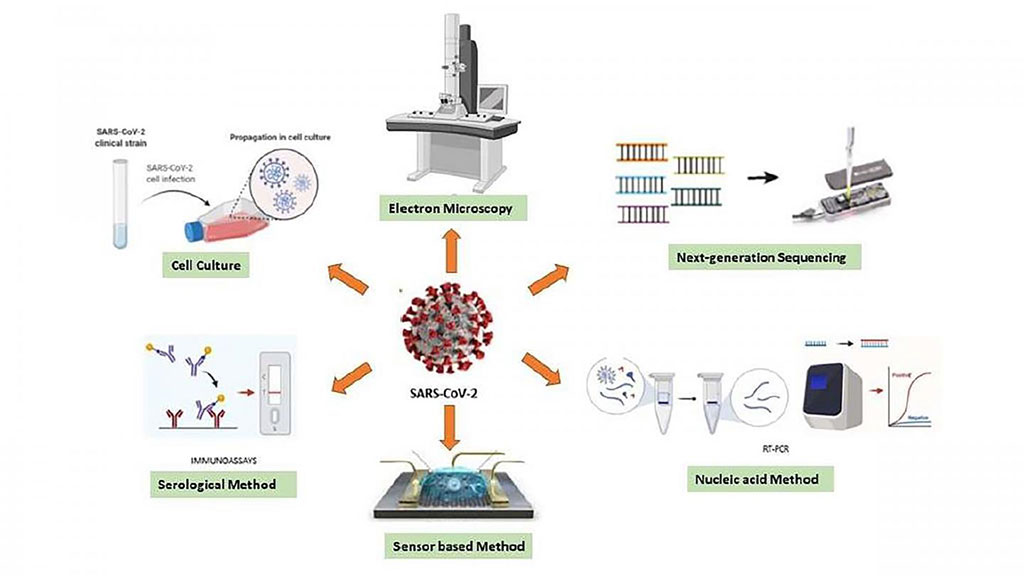
Nano-Based Sensor Technologies Could Make It Possible to Detect COVID-19 at Any Disease Stage, Say Researchers
Despite various diagnostic techniques for COVID-19, no single test is available for the entire stage of the disease, although nano-based sensor technologies could make it possible to detect the attack at any stage of the disease, according to researchers. More...02 Dec 2020
In Other News
GENETWORx Labs Launches New Breakthrough Diagnostic Flu A-B/COVID-19/RSV Combination Test
Bio-Rad Launches SARS CoV-2 Standard for Coronavirus (COVID-19) Testing
Fastest Ever Robotics-Driven COVID-19 Mass Testing Platform Gives 99% Accurate Results
New High-Throughput RT-PCR Testing System for SARS-CoV-2 Can Process Up to 35,000 Tests per Day
COVID-19 Rapid Breath Test Uses Exhaled VOCs in Human Breath as Biomarkers of SARS-CoV-2
Printable POC SARS-CoV-2 Antibody Test for COVID-19 Could Produce Results in Real Time
Machine Learning Algorithm for Identifying Single Virus Particles Could Lead to More Accurate, Fast COVID-19 Test
Self-Swab Kit for COVID-19 Test Granted Emergency Use Authorization by FDA
First-Ever COVID-19 Severity Test to Triage Patients Quickly and Accurately
RapidRona Self-Collection COVID-19 Kit Secures FDA Emergency Use Authorization
FDA Grants EUA to One of the First Tests That Measures Specific Levels of COVID-19 Neutralizing Antibodies
Mast Group Launches New ESPLINE SARS-CoV-2 Lateral Flow Antigen Test
Beckman Coulter Launches Semi-Quantitative Access SARS-CoV-2 IgG II Antibody Test
Cepheid Receives CE-IVD Registration for SARS-CoV-2, Flu A, Flu B and RSV Combination Test
Ortho's CE-Marked VITROS SARS-CoV-2 Antigen Test Detects Asymptomatic COVID-19 Individuals
First Hybridization Capture-Based NGS Assay for Characterization and Surveillance of SARS-CoV-2 Launched
FDA Authorizes First COVID-19 Test for Self-Testing at Home
Siemens Becomes First Leading Diagnostics Company to Offer Quantitative COVID-19 Test for Measuring Neutralizing Antibodies
Bioneer’s Combination Tests for SARS-CoV-2 and Influenza Receive CE-IVD Marking
Mobile Mini-Laboratory System Identifies SARS-CoV-2 Virus in 40 Minutes and Conducts 60 Tests per Day
ELITechGroup Submits SARS-CoV-2 Plus ELITe MGB Assay for FDA Emergency Use Authorization
Virusight Diagnostic’s AI Solution for COVID-19 1-Second Test Demonstrates Excellent Correlation to PCR
Study Finds New Saliva-Based Antibody Test for SARS-CoV-2 Highly Accurate











