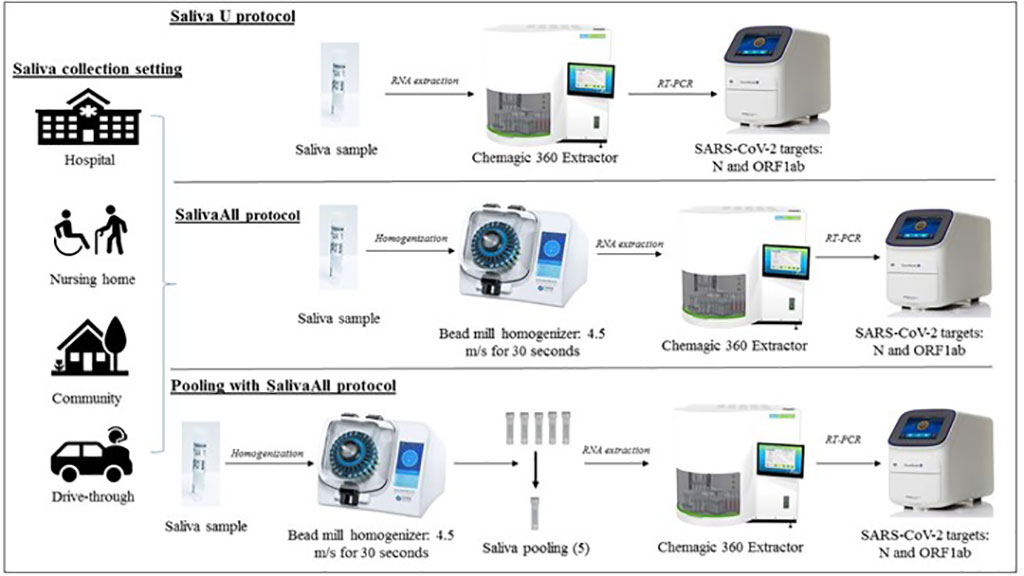
Innovative Protocol That Processes Saliva Samples with Bead Mill Homogenizer Before RT-PCR Testing Improves COVID-19 Detection Rate
The addition of a simple processing step to saliva samples before testing may improve COVID-19 detection rate, eliminate the challenges of nasopharyngeal testing, and facilitate mass surveillance, according to researchers. More...14 Jun 2021

Increasing Efficacy of Antibody Tests Propelling Growth of Global Immunoassay Market Amidst COVID-19 Pandemic
The growth of the global immunoassay market is being propelled by the drive to increase the efficacy of antibody tests amidst the COVID-19 pandemic, led further by the growing use of immunoassay methods in diagnosis of various autoimmune diseases. More...11 Jun 2021
CRISPR-Based COVID-19 Rapid Test Accurately Detects Coronavirus in as Little as One Minute
Researchers have repurposed a CRISPR system to make a rapid, accurate COVID-19 test that can accurately detect even relatively small amounts of coronavirus in patient samples in less than 30 minutes and sometimes in as little as one minute. More...10 Jun 2021

Handheld RT-PCR System Combined with Saliva-Based Test Brings Mobility to COVID-19 Testing and Reduces Cost
COVID-19 testing has been made easier and more accessible with the US FDA emergency use authorization (EUA) of the combination of SalivaDirect developed by Yale School of Public Health (New Haven, CT, USA) with Ubiquitome Limited’s (Auckland, New Zealand) handheld Liberty16 mobile real time-PCR. More...09 Jun 2021
OraSure’s Easy and Intuitive ‘Swab, Swirl and See’ COVID-19 Tests Granted FDA Emergency Use Authorization
OraSure Technologies, Inc. (Bethlehem, PA, USA) has received Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) for its InteliSwab COVID-19 rapid antigen tests which detect active COVID-19 infection. More...08 Jun 2021
New Method for Rapid, Accurate Detection of Viruses Could Speed Up COVID-19 Testing
Researchers have developed a method for rapid, accurate detection of viruses that is four times faster than conventional PCR methods, is highly specific, sensitive, and resistant to inhibitors and could speed up COVID-19 testing. More...08 Jun 2021
In Other News
Rapid Blood Test Could Confirm COVID-19 Vaccination in Minutes
COVID-19 Transformation to Help Global qPCR and dPCR Instrumentation Market Reach USD 9.2 Billion by 2026
Eurofins Launches New Multiplex PCR Assay for Rapid Detection of B.1.617 Kappa SARS-CoV-2 Variant
COVID-19 Rapid Antigen Test Provides End-to-End Digital Screening Solution
New Electronic-Based ‘Nose’ Detector Sniffs out COVID-19-Infected People in 80 Seconds
New, Fast, Portable Saliva Screening Test Uses Infrared Light Technology to Confirm SARS-CoV-2 Infection
Microfluidics-Based POC Diagnostic Devices for COVID-19 More Accurate than Lateral Flow Assays, Finds Frost & Sullivan
AI with Swarm Intelligence Detects COVID-19 in Data Stored in Decentralized Fashion
Ultrafast, On-Chip PCR that Detects SARS-CoV-2 in Only 8 Minutes Could Speed COVID-19 Diagnosis
Next Generation Sequencing Testing Protocol for SARS-CoV-2 Can Process Tens of Thousands of Samples in Less Than 48 Hours
Seegene Unveils Exclusive Diagnostic System for Diagnosing COVID-19 Variants
Diasorin Launches New Assay for Rapid Identification of SARS-CoV-2 Variants
Ortho Launches First Quantitative COVID-19 IgG Spike Antibody Test and New Nucleocapsid Antibody Test
Using CLEIA for Screening and RT-PCR for Confirmation Increases Accuracy of COVID-19 Diagnosis, Finds New Study
Roche Receives FDA EUA for Testing of Asymptomatic People with High-Throughput, Highly Sensitive Cobas SARS-CoV-2 Test
Beckman Coulter Launches Fully Quantitative and Automated COVID-19 IgG Test to Assess Antibody Immune Response Levels
New Antigen-Based COVID-19 Rapid Test Format Analyzes 500 Samples per Hour and Detects SARS-CoV-2 Infection in 10 Minutes
Portable, Battery-Powered Device Combined with Rapid, Highly Sensitive and Accurate Assay Enables COVID-19 Testing Anytime, Anywhere
New Microchip Real-Time Technology Platform for COVID-19 Testing Offers Alternative to Gold-Standard RT-QPCR Tests
Superfast, Portable COVID-19 Testing Method Detects SARS-CoV-2 Within One Second
Novel X-Ray Imaging Technique Reveals Best SARS-CoV-2 Antibodies for Developing Reliable, Rapid COVID-19 Tests
Oxford University and Oracle Create Machine Intelligence-Powered Global Pathogen Analysis System to Speed Identification of COVID-19 Variants
Testing Tool that Accurately Distinguishes Between Viral and Bacterial Infection for Respiratory Illness Could Aid Fight Against COVID-19











