Using CLEIA for Screening and RT-PCR for Confirmation Increases Accuracy of COVID-19 Diagnosis, Finds New Study
By LabMedica International staff writers
Posted on 21 May 2021
Scientists have shown that an antigen-based test for quantifying SARS-CoV-2 in saliva samples is simple, rapid, and more conducive for mass-screening, suggesting that combined CLEIA and RT-PCR testing on saliva samples increases the accuracy of diagnosis.Posted on 21 May 2021
A team of scientists from Hokkaido University (Hokkaido, Japan) used a novel antigen-based kit, Lumipulse SARS-CoV-2 Ag kit (Lumipulse), developed by Fujirebio Europe (Ghent, Belgium) to quantitatively measure the viral antigen in biological samples within 35 minutes. The team used the antigen kit to detect SARS-CoV-2 in saliva samples, and assess the efficiency and accuracy of the test compared to RT-PCR. Their findings show that the antigen detection kit, which is used to perform chemiluminescent enzyme immunoassay (CLEIA), can rapidly detect SARS-CoV-2 with good accuracy in these samples.
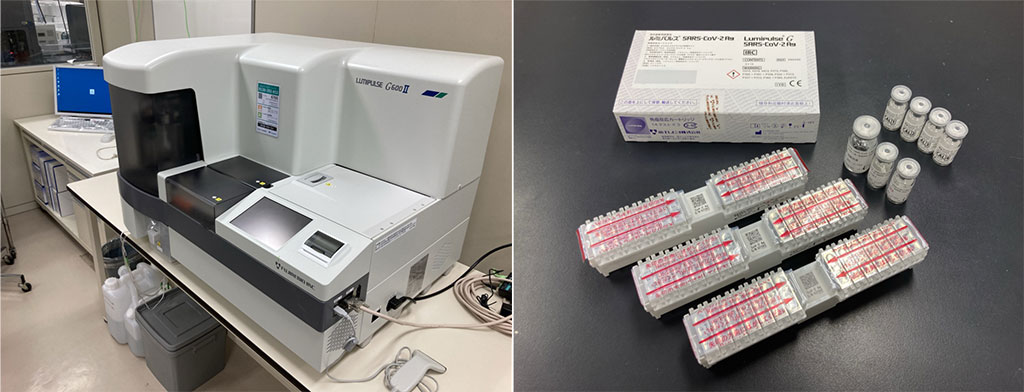
Image: The Lumipulse G600II instrument (left) and the Lumipulse® SARS-CoV-2 Ag kit (right), both manufactured by Fujirebio, which were used in this study for the quantification of SARS-CoV-2 in saliva samples (Photo courtesy of Shinichi Fujisawa)
The scientists tested 2056 individuals from three cohorts: patients with clinically confirmed COVID-19, individuals who had contacted patients with COVID-19, and individuals tested on arrival at Tokyo and Kansai International Airports. Saliva samples were collected from all individuals and used for RT-PCR tests as well as CLEIA using Lumipulse. The results of both were compared to determine the usefulness of CLEIA. The scientists found that CLEIA is a reliable test, as it correlates well with RT-PCR. CLEIA alone can be used to detect SARS-CoV-2 within an hour; however, using CLEIA for screening and RT-PCR for confirmation increases the accuracy of diagnosis.
The benefit of using saliva samples is the ease of collection: it is quick and can be collected by the individuals being tested, reducing the risk that healthcare workers are exposed to the virus. Furthermore, self-collection of saliva allows multiple samples to be collected simultaneously for expeditious screening of visitors at large gatherings. Combined CLEIA and RT-PCR testing on saliva samples has already been implemented at Japanese airport quarantines, and the scientists have recommended adopting it at a wider scale to rapidly screen for SARS-CoV-2.
Related Links:
Hokkaido University
Fujirebio Europe














