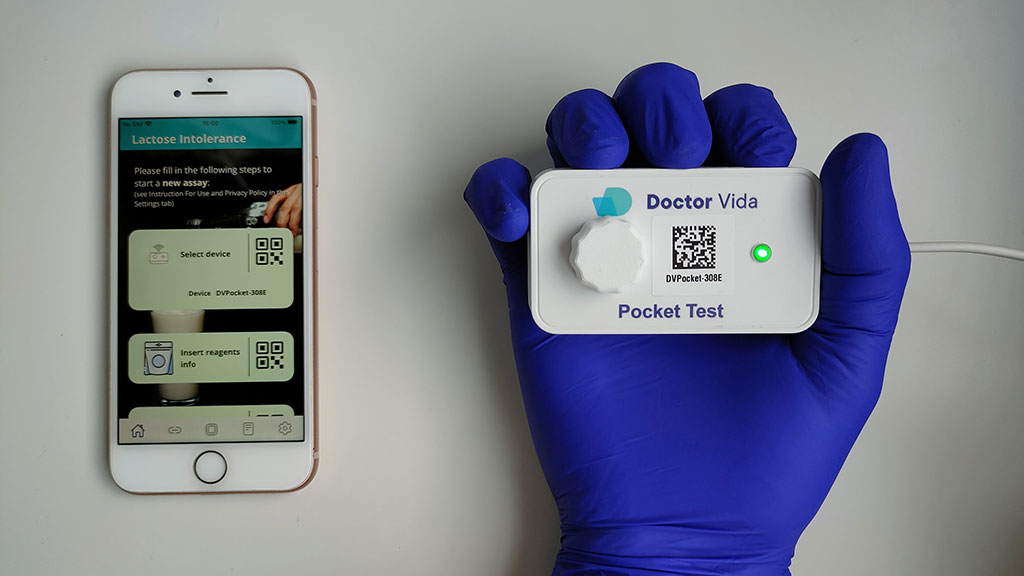
First POC Genetic Test Diagnoses Lactose Intolerance Near Patient in Less Than 90 Minutes
|
By LabMedica International staff writers Posted on 05 Sep 2023 |
Lactose intolerance is a medical condition marked by difficulty in digesting lactose due to reduced activity or synthesis of the enzyme lactase, which breaks down lactose into its basic sugars. A specific genetic variant called the −13910 C/T single nucleotide polymorphism within the MCM6 gene is linked to lactase persistence or non-persistence traits in the Caucasian population. Diagnosing lactose intolerance is crucial for implementing dietary adjustments or appropriate treatments. Now, a new point-of-care isothermal lab-on-phone lactose intolerance assay rapidly diagnoses primary lactose intolerance using direct buccal swabs or capillary blood samples with high accuracy and at low cost.
STAB VIDA (Caparica, Portugal) has developed an isothermal lab-on-phone lactose intolerance CE-IVD (In Vitro Diagnostic) marked point-of-care diagnostic assay called STAB VIDA Lda which is based on the loop-mediated isothermal amplification (LAMP) methodology followed by mutation probe-based melting curve analysis. The assay is automatically processed in real time using the Doctor Vida system, an inexpensive and portable device controlled through a user-friendly mobile app. This CE-IVD portable, handheld device facilitates isothermal nucleic acid amplification for a wide range of genetic biomarkers. Combining efficiency, accuracy, and affordability, this compact lab-on-phone lactose intolerance detection assay can prove highly valuable in managing symptoms for individuals with lactose intolerance. Moreover, it differentiates between primary and secondary forms of hypolactasia, with the former arising from genetic mutations in the enhancer region and the latter resulting from small intestine damage.
In a study, researchers evaluated the POC isothermal lab-on-phone lactose intolerance assay using buccal swabs and capillary blood samples, comparing its performance with the Sanger sequencing assay that examined the -13,910 C/T SNP in blood samples. The assay's detection limit was also determined across different sample concentrations. The findings showed that the STAB VIDA Lda isothermal lactose intolerance assay effectively identified the -13910 C/T SNP with a limit of detection of five cells per assay, achieving 98.41% accuracy for buccal swabs and 100% accuracy for capillary blood samples. The entire process, from sample collection to result retrieval, was completed in just 90 minutes, with merely two minutes of hands-on time. The detection limit achieved by the STAB VIDA Lda assay was comparable to established gold-standard methods like Sanger sequencing and PCR-based assays.
Related Links:
STAB VIDA
Latest Molecular Diagnostics News
- Diagnostic Device Predicts Treatment Response for Brain Tumors Via Blood Test
- Blood Test Detects Early-Stage Cancers by Measuring Epigenetic Instability
- Two-in-One DNA Analysis Improves Diagnostic Accuracy While Saving Time and Costs
- “Lab-On-A-Disc” Device Paves Way for More Automated Liquid Biopsies
- New Tool Maps Chromosome Shifts in Cancer Cells to Predict Tumor Evolution
- Blood Test Identifies Inflammatory Breast Cancer Patients at Increased Risk of Brain Metastasis
- Newly-Identified Parkinson’s Biomarkers to Enable Early Diagnosis Via Blood Tests
- New Blood Test Could Detect Pancreatic Cancer at More Treatable Stage
- Liquid Biopsy Could Replace Surgical Biopsy for Diagnosing Primary Central Nervous Lymphoma
- New Tool Reveals Hidden Metabolic Weakness in Blood Cancers
- World's First Blood Test Distinguishes Between Benign and Cancerous Lung Nodules
- Rapid Test Uses Mobile Phone to Identify Severe Imported Malaria Within Minutes
- Gut Microbiome Signatures Predict Long-Term Outcomes in Acute Pancreatitis
- Blood Test Promises Faster Answers for Deadly Fungal Infections
- Blood Test Could Detect Infection Exposure History
- Urine-Based MRD Test Tracks Response to Bladder Cancer Surgery
Channels
Clinical Chemistry
view channel
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read more
Extracellular Vesicles Linked to Heart Failure Risk in CKD Patients
Chronic kidney disease (CKD) affects more than 1 in 7 Americans and is strongly associated with cardiovascular complications, which account for more than half of deaths among people with CKD.... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more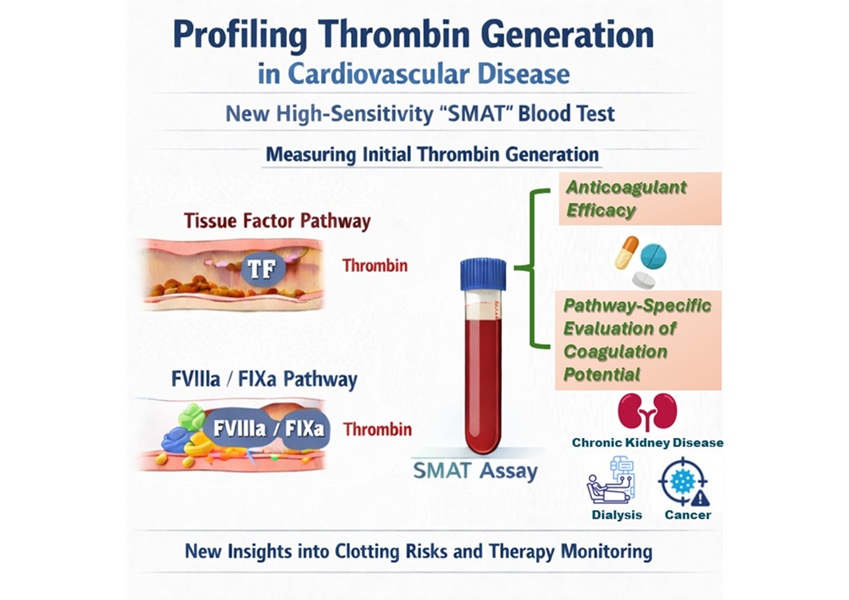
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read moreAI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
Infections caused by the pathogenic yeast Candida auris pose a significant threat to hospitalized patients, particularly those with weakened immune systems or those who have invasive medical devices.... Read morePathology
view channel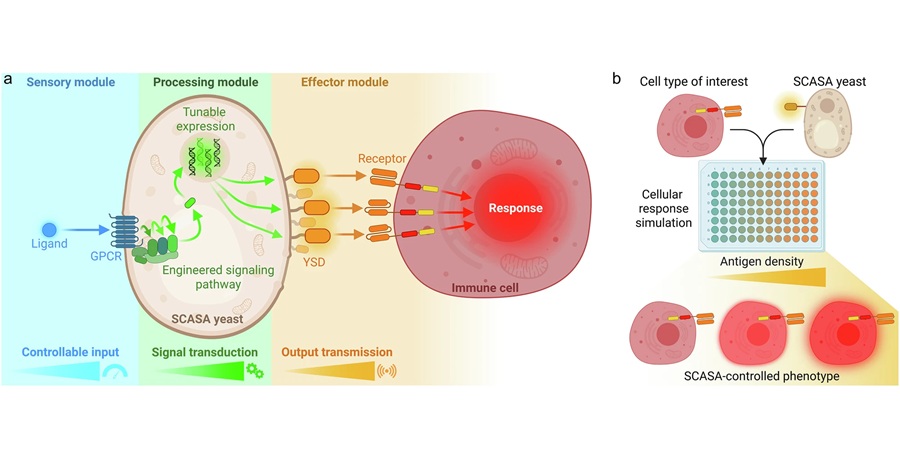
Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Developing new cancer immunotherapies is a slow, costly, and high-risk process, particularly for CAR T cell treatments that must precisely recognize cancer-specific antigens. Small differences in tumor... Read more
First-Of-Its-Kind Test Identifies Autism Risk at Birth
Autism spectrum disorder is treatable, and extensive research shows that early intervention can significantly improve cognitive, social, and behavioral outcomes. Yet in the United States, the average age... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more
AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
Pre-eclampsia and anemia are major contributors to maternal and child mortality worldwide, together accounting for more than half a million deaths each year and leaving millions with long-term health complications.... Read moreIndustry
view channelNew Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
Mass spectrometry is a powerful analytical technique that identifies and quantifies molecules based on their mass and electrical charge. Its high selectivity, sensitivity, and accuracy make it indispensable... Read more
AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
Noul Co., a Korean company specializing in AI-based blood and cancer diagnostics, announced it will supply its intelligence (AI)-based miLab CER cervical cancer diagnostic solution to Mexico under a multi‑year... Read more
Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
Diasorin (Saluggia, Italy) has entered into an exclusive distribution agreement with Fisher Scientific, part of Thermo Fisher Scientific (Waltham, MA, USA), for the LIAISON NES molecular point-of-care... Read more