Hologic to Acquire Mobidiag to Strengthen Diagnostic Testing Business
|
By LabMedica International staff writers Posted on 09 Apr 2021 |

Image: The Novodiag platform combines real-time PCR and microarray capabilities to provide high-level multiplexing (Photo courtesy of Mobidiag Ltd.)
Hologic, Inc. (Marlborough, MA, USA) has signed a definitive agreement to acquire Mobidiag Ltd. (Espoo, Finland) in a USD 795 million transaction that will accelerate the global growth of Mobidiag’s differentiated molecular platform, which offers ease of use, multiplex capability and rapid turnaround time.
Mobidiag, a privately held, commercial-stage developer of molecular diagnostic tests and instrumentation, develops and markets PCR (polymerase chain reaction)-based tests for acute care conditions such as gastrointestinal and respiratory infections, antimicrobial resistance management, and healthcare associated infections. The Amplidiag and Novodiag platforms are automated instruments that deliver rapid turnaround times ranging from 50 minutes to two hours.
The Novodiag platform combines real-time PCR and microarray capabilities to provide high-level multiplexing. Multiplexing enables multiple pathogens to be identified in a single sample, streamlining workflows for laboratories and providing rapid results to physicians. For example, gastrointestinal infections commonly present with similar or identical symptoms but can be caused by more than 25 organisms. Highly multiplexed assays allow clinicians to identify which organism is responsible for an infection quickly, accurately and efficiently.
“Acquiring Mobidiag will further strengthen our international and diagnostics businesses by enabling us to expand into the large, fast-growing acute care adjacency with a near-patient testing solution that offers ease of use, multiplex capability and rapid turnaround time,” said Jan Verstreken, group president, international at Hologic. “We believe that Mobidiag has developed a differentiated platform that addresses many of the historical challenges of multiplexed point-of-care molecular testing.”
“One of our key goals is to use our strong cash flow to create a larger, faster growing company for a post-pandemic world,” said Steve MacMillan, Hologic’s chairman, president and chief executive officer. “Mobidiag provides an exceptional new growth platform, which will generate long-term value by enabling us to enter the acute care market, which is expected to roughly double in the next five years, with a differentiated, highly competitive solution.”
“We are very excited to join Hologic’s diagnostic business,” said Tuomas Tenkanen, Mobidiag’s chief executive officer. “Hologic’s commercial expertise and scale will drive broader market adoption of our products, and their established US regulatory and market development capabilities will accelerate the introduction of our products and maximize their potential in the United States.”
Related Links:
Hologic, Inc.
Mobidiag Ltd.
Mobidiag, a privately held, commercial-stage developer of molecular diagnostic tests and instrumentation, develops and markets PCR (polymerase chain reaction)-based tests for acute care conditions such as gastrointestinal and respiratory infections, antimicrobial resistance management, and healthcare associated infections. The Amplidiag and Novodiag platforms are automated instruments that deliver rapid turnaround times ranging from 50 minutes to two hours.
The Novodiag platform combines real-time PCR and microarray capabilities to provide high-level multiplexing. Multiplexing enables multiple pathogens to be identified in a single sample, streamlining workflows for laboratories and providing rapid results to physicians. For example, gastrointestinal infections commonly present with similar or identical symptoms but can be caused by more than 25 organisms. Highly multiplexed assays allow clinicians to identify which organism is responsible for an infection quickly, accurately and efficiently.
“Acquiring Mobidiag will further strengthen our international and diagnostics businesses by enabling us to expand into the large, fast-growing acute care adjacency with a near-patient testing solution that offers ease of use, multiplex capability and rapid turnaround time,” said Jan Verstreken, group president, international at Hologic. “We believe that Mobidiag has developed a differentiated platform that addresses many of the historical challenges of multiplexed point-of-care molecular testing.”
“One of our key goals is to use our strong cash flow to create a larger, faster growing company for a post-pandemic world,” said Steve MacMillan, Hologic’s chairman, president and chief executive officer. “Mobidiag provides an exceptional new growth platform, which will generate long-term value by enabling us to enter the acute care market, which is expected to roughly double in the next five years, with a differentiated, highly competitive solution.”
“We are very excited to join Hologic’s diagnostic business,” said Tuomas Tenkanen, Mobidiag’s chief executive officer. “Hologic’s commercial expertise and scale will drive broader market adoption of our products, and their established US regulatory and market development capabilities will accelerate the introduction of our products and maximize their potential in the United States.”
Related Links:
Hologic, Inc.
Mobidiag Ltd.
Latest Industry News
- AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
- New Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
- Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
- WHX Labs Dubai to Gather Global Experts in Antimicrobial Resistance at Inaugural AMR Leaders’ Summit
- BD and Penn Institute Collaborate to Advance Immunotherapy through Flow Cytometry
- Abbott Acquires Cancer-Screening Company Exact Sciences
- Roche and Freenome Collaborate to Develop Cancer Screening Tests
- Co-Diagnostics Forms New Business Unit to Develop AI-Powered Diagnostics
- Qiagen Acquires Single-Cell Omics Firm Parse Biosciences
- Puritan Medical Products Showcasing Innovation at AMP2025 in Boston
- Advanced Instruments Merged Under Nova Biomedical Name
- Bio-Rad and Biodesix Partner to Develop Droplet Digital PCR High Complexity Assays
- Hologic to be Acquired by Blackstone and TPG
- Bio-Techne and Oxford Nanopore to Accelerate Development of Genetics Portfolio
- Terumo BCT and Hemex Health Collaborate to Improve Access to Testing for Hemoglobin Disorders
- Revvity and Sanofi Collaborate on Program to Revolutionize Early Detection of Type 1 Diabetes
Channels
Clinical Chemistry
view channel
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read more
Extracellular Vesicles Linked to Heart Failure Risk in CKD Patients
Chronic kidney disease (CKD) affects more than 1 in 7 Americans and is strongly associated with cardiovascular complications, which account for more than half of deaths among people with CKD.... Read moreMolecular Diagnostics
view channel
Diagnostic Device Predicts Treatment Response for Brain Tumors Via Blood Test
Glioblastoma is one of the deadliest forms of brain cancer, largely because doctors have no reliable way to determine whether treatments are working in real time. Assessing therapeutic response currently... Read more
Blood Test Detects Early-Stage Cancers by Measuring Epigenetic Instability
Early-stage cancers are notoriously difficult to detect because molecular changes are subtle and often missed by existing screening tools. Many liquid biopsies rely on measuring absolute DNA methylation... Read more
“Lab-On-A-Disc” Device Paves Way for More Automated Liquid Biopsies
Extracellular vesicles (EVs) are tiny particles released by cells into the bloodstream that carry molecular information about a cell’s condition, including whether it is cancerous. However, EVs are highly... Read more
Blood Test Identifies Inflammatory Breast Cancer Patients at Increased Risk of Brain Metastasis
Brain metastasis is a frequent and devastating complication in patients with inflammatory breast cancer, an aggressive subtype with limited treatment options. Despite its high incidence, the biological... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more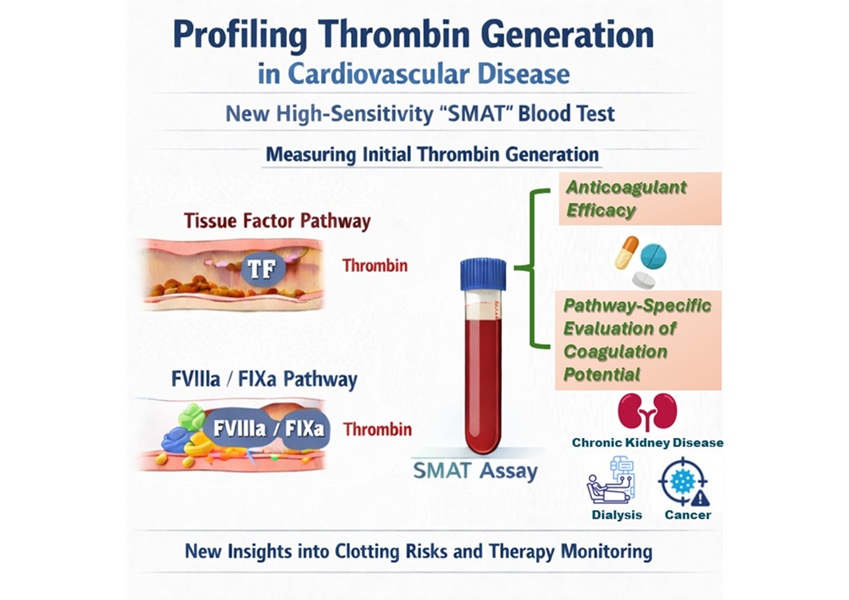
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read moreAI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
Infections caused by the pathogenic yeast Candida auris pose a significant threat to hospitalized patients, particularly those with weakened immune systems or those who have invasive medical devices.... Read morePathology
view channel
Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Developing new cancer immunotherapies is a slow, costly, and high-risk process, particularly for CAR T cell treatments that must precisely recognize cancer-specific antigens. Small differences in tumor... Read more
First-Of-Its-Kind Test Identifies Autism Risk at Birth
Autism spectrum disorder is treatable, and extensive research shows that early intervention can significantly improve cognitive, social, and behavioral outcomes. Yet in the United States, the average age... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more