Molecular Assays Developed for Hantavirus Pulmonary Syndrome
|
By LabMedica International staff writers Posted on 16 Jan 2020 |
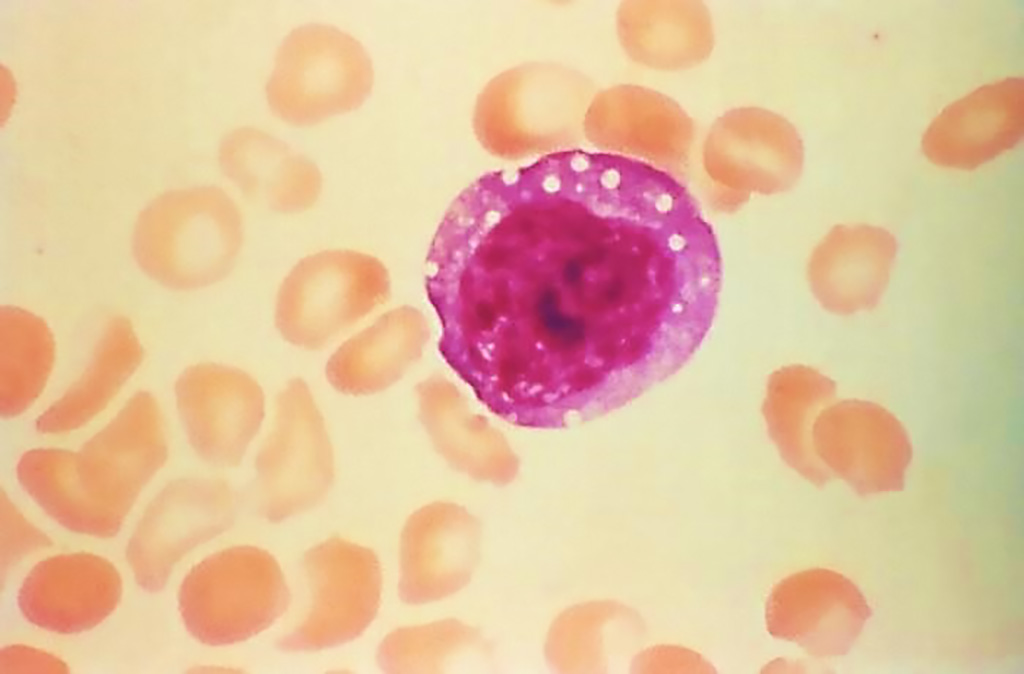
Image: This micrograph depicts an atypical, enlarged lymphocyte found in the blood smear from a hantavirus pulmonary syndrome (HPS) patient. This large atypical lymphocyte is an example of one of the laboratory findings, which when combined with a bandemia, i.e., immature white blood cells, and dropping platelet count, is characteristic of HPS (Photo courtesy of Centers for Disease Control and Prevention)
Hantavirus Pulmonary Syndrome (HPS) is a serious and often fatal disease caused by viruses known as hantaviruses. These anthropozoonotic diseases comprising of two clinical entities: Hemorrhagic Fever with Renal Syndrome (HFRS), which occurs in the Old World, and Hantavirus Pulmonary Syndrome (HPS) in the New World.
Laboratory diagnosis of HPS is often conducted by enzyme-linked immunosorbent assay (ELISA) for detection of immunoglobulin G (IgG) and IgM specific antibodies in serum or blood of suspected patients. Given that IgM antibodies are detectable early in the disease, IgM ELISA is considered the reference method for HPS diagnostics.
Scientists at the Evandro Chagas Institute (Ananindeua, Brazil) have developed real-time polymerase chain reaction (RT-qPCR) and semi-nested RT-qPCR assays for the diagnosis of HPS. The assays were developed for detection and quantification of four hantaviruses strains circulating in the Brazilian Amazon. assays were used to test two groups of samples: one comprising of 50 patients with disease and other containing samples from 50 healthy individuals according to IgM-ELISA results. A third group of 27 samples infected with other pathogens were tested for specificity analysis.
A consensus region in the N gene of these hantaviruses was used to design the primer sets and a hydrolysis probe. In vitro transcribed RNA was diluted in standards with known concentration. MS2 bacteriophage RNA was detected together with hantavirus RNA as an exogenous control in a duplex reaction. RT-qPCR efficiency was around 100% and the limit of detection was 0.9 copies/μL of RNA for RT-qPCR and 10 copies/μL of RNA for Semi-nested RT-PCR. There was no amplification of either negative samples or samples positive to other pathogens. Sample reactions were performed in triplicate in ViiA7 qPCR System (Applied Biosystems, Thermo-Fisher Scientific, Waltham, MA, USA).
The investigators reported that the RT-qPCR was more sensitive than semi-nested RT-PCR, being able to detect three samples undetected by conventional RT-PCR. RT-qPCR clinical sensitivity, specificity and general accuracy values were 92.5%, 100% and 97.63%, respectively. Taken together, these results indicate that both RT-qPCR and Semi-nested RT-PCR assays are efficient, sensitive and specific tools for genome detection of hantaviruses circulating in the Amazon region. In addition to being more sensitive, RT-qPCR offers other advantages such as agility in generating results, about three times faster than conventional assays and automation capability which leads to lower risk of contamination and greater reproducibility. The study was published on December 26, 2019 in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
Evandro Chagas Institute
Applied Biosystems
Laboratory diagnosis of HPS is often conducted by enzyme-linked immunosorbent assay (ELISA) for detection of immunoglobulin G (IgG) and IgM specific antibodies in serum or blood of suspected patients. Given that IgM antibodies are detectable early in the disease, IgM ELISA is considered the reference method for HPS diagnostics.
Scientists at the Evandro Chagas Institute (Ananindeua, Brazil) have developed real-time polymerase chain reaction (RT-qPCR) and semi-nested RT-qPCR assays for the diagnosis of HPS. The assays were developed for detection and quantification of four hantaviruses strains circulating in the Brazilian Amazon. assays were used to test two groups of samples: one comprising of 50 patients with disease and other containing samples from 50 healthy individuals according to IgM-ELISA results. A third group of 27 samples infected with other pathogens were tested for specificity analysis.
A consensus region in the N gene of these hantaviruses was used to design the primer sets and a hydrolysis probe. In vitro transcribed RNA was diluted in standards with known concentration. MS2 bacteriophage RNA was detected together with hantavirus RNA as an exogenous control in a duplex reaction. RT-qPCR efficiency was around 100% and the limit of detection was 0.9 copies/μL of RNA for RT-qPCR and 10 copies/μL of RNA for Semi-nested RT-PCR. There was no amplification of either negative samples or samples positive to other pathogens. Sample reactions were performed in triplicate in ViiA7 qPCR System (Applied Biosystems, Thermo-Fisher Scientific, Waltham, MA, USA).
The investigators reported that the RT-qPCR was more sensitive than semi-nested RT-PCR, being able to detect three samples undetected by conventional RT-PCR. RT-qPCR clinical sensitivity, specificity and general accuracy values were 92.5%, 100% and 97.63%, respectively. Taken together, these results indicate that both RT-qPCR and Semi-nested RT-PCR assays are efficient, sensitive and specific tools for genome detection of hantaviruses circulating in the Amazon region. In addition to being more sensitive, RT-qPCR offers other advantages such as agility in generating results, about three times faster than conventional assays and automation capability which leads to lower risk of contamination and greater reproducibility. The study was published on December 26, 2019 in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
Evandro Chagas Institute
Applied Biosystems
Latest Microbiology News
- Blood-Based Viral Signature Identified in Crohn’s Disease
- Hidden Gut Viruses Linked to Colorectal Cancer Risk
- Three-Test Panel Launched for Detection of Liver Fluke Infections
- Rapid Test Promises Faster Answers for Drug-Resistant Infections
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- New UTI Diagnosis Method Delivers Antibiotic Resistance Results 24 Hours Earlier
- Breakthroughs in Microbial Analysis to Enhance Disease Prediction
- Blood-Based Diagnostic Method Could Identify Pediatric LRTIs
- Rapid Diagnostic Test Matches Gold Standard for Sepsis Detection
- Rapid POC Tuberculosis Test Provides Results Within 15 Minutes
- Rapid Assay Identifies Bloodstream Infection Pathogens Directly from Patient Samples
- Blood-Based Molecular Signatures to Enable Rapid EPTB Diagnosis
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read moreMicrobiology
view channel
Blood-Based Viral Signature Identified in Crohn’s Disease
Crohn’s disease is a chronic inflammatory intestinal disorder affecting approximately 0.4% of the European population, with symptoms and progression that vary widely. Although viral components of the microbiome... Read more
Hidden Gut Viruses Linked to Colorectal Cancer Risk
Colorectal cancer (CRC) remains a leading cause of cancer mortality in many Western countries, and existing risk-stratification approaches leave substantial room for improvement. Although age, diet, and... Read morePathology
view channel
Molecular Imaging to Reduce Need for Melanoma Biopsies
Melanoma is the deadliest form of skin cancer and accounts for the vast majority of skin cancer-related deaths. Because early melanomas can closely resemble benign moles, clinicians often rely on visual... Read more
Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
Urine testing is a critical, non-invasive diagnostic tool used to detect conditions such as pregnancy, urinary tract infections, metabolic disorders, cancer, and kidney disease. However, contaminated or... Read moreTechnology
view channel
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read more
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more



















