Liquid Biopsy Detects Breast Cancer Residual Disease
|
By LabMedica International staff writers Posted on 02 Jan 2020 |
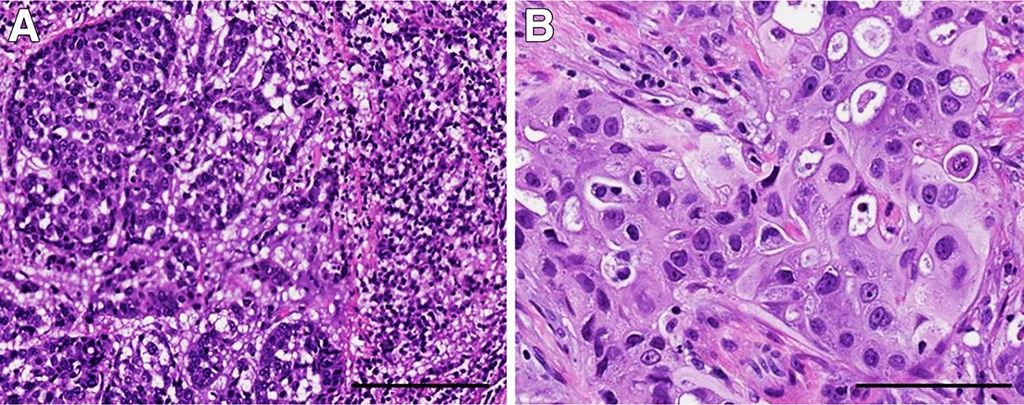
Image: Morphologic variants of triple-negative breast cancer (TNBC) with different genetic alterations. A: TNBC with basal-like histologic features containing a prominent stromal lymphocytic infiltrate; this tumor had MYC amplification. B: TNBC with apocrine differentiation and a PI3KCA mutation. The tumor cells have abundant eosinophilic cytoplasm, round nuclei, and prominent nucleoli (Photo courtesy of Geisel School of Medicine).
The concept of circulating DNA or tumor cells to identify patients with residual cancer present after early-stage, putatively curative treatment has made rapid strides across multiple tumor types since some of the first promising tests in colorectal cancer.
Triple-negative breast cancers, unlike some other breast cancer types, carry significant risk of recurrence even when diagnosed at early stages. In addition, they lack the growing slate of targeted treatment options available to other molecular subsets and because of this, standard of care is limited to chemotherapy, radiation, and surgery.
Scientists from the Indiana University School of Medicine (Indianapolis, IN, USA) and their colleagues analyzed retrospective plasma samples that had been collected from patients enrolled in the Phase II BRE12-158 clinical trial, which studied genomically directed therapy versus physician’s choice of treatment after preoperative chemotherapy in patients with high-risk triple-negative breast cancer.
The trial enrolled 196 women in total, 142 of whom had circulating tumor DNA (ctDNA) sequencing performed using the FoundationOne Liquid Test (Foundation Medicine, Cambridge, MA, USA) and enough clinical follow-up to study. Testing identified mutated ctDNA in 90 of the patients, about 60 %, with TP53 being the most commonly mutated gene, followed by others that are commonly associated with breast cancer.
At 17.2 months of follow up, the patients in whom ctDNA had been detected had significantly inferior distant disease free survival (DDFS) compared to those who didn't. The group showing circulating mutations survived without distant recurrences 32.5 months on average, while patients without ctDNA had not reached a median. At 24 months, the DDFS rate was 56% for ctDNA-positive patients, compared with 81% in ctDNA-negative patients. By combining ctDNA and circulating tumor cell detection boosted this even further. Patients who were double positive (having both ctDNA and circulating tumor cells present) had a two-year DDFS of just 52% compared to 89% for double negatives.
Milan Radovich, PhD, an associate professor and first author of the study said, “With neoadjuvant chemotherapy about one third of triple-negative patients achieve a state of pathologic complete response, in which there is no evidence of their tumor once surgeons go in to remove it. This subgroup has much better outcomes than the two thirds who still have residual disease after neoadjuvant chemo.” The study was presented at the San Antonio Breast Cancer Symposium held December 10 - 14, 2019, in San Antonio, TX, USA.
Related Links:
Indiana University School of Medicine
Foundation Medicine
Triple-negative breast cancers, unlike some other breast cancer types, carry significant risk of recurrence even when diagnosed at early stages. In addition, they lack the growing slate of targeted treatment options available to other molecular subsets and because of this, standard of care is limited to chemotherapy, radiation, and surgery.
Scientists from the Indiana University School of Medicine (Indianapolis, IN, USA) and their colleagues analyzed retrospective plasma samples that had been collected from patients enrolled in the Phase II BRE12-158 clinical trial, which studied genomically directed therapy versus physician’s choice of treatment after preoperative chemotherapy in patients with high-risk triple-negative breast cancer.
The trial enrolled 196 women in total, 142 of whom had circulating tumor DNA (ctDNA) sequencing performed using the FoundationOne Liquid Test (Foundation Medicine, Cambridge, MA, USA) and enough clinical follow-up to study. Testing identified mutated ctDNA in 90 of the patients, about 60 %, with TP53 being the most commonly mutated gene, followed by others that are commonly associated with breast cancer.
At 17.2 months of follow up, the patients in whom ctDNA had been detected had significantly inferior distant disease free survival (DDFS) compared to those who didn't. The group showing circulating mutations survived without distant recurrences 32.5 months on average, while patients without ctDNA had not reached a median. At 24 months, the DDFS rate was 56% for ctDNA-positive patients, compared with 81% in ctDNA-negative patients. By combining ctDNA and circulating tumor cell detection boosted this even further. Patients who were double positive (having both ctDNA and circulating tumor cells present) had a two-year DDFS of just 52% compared to 89% for double negatives.
Milan Radovich, PhD, an associate professor and first author of the study said, “With neoadjuvant chemotherapy about one third of triple-negative patients achieve a state of pathologic complete response, in which there is no evidence of their tumor once surgeons go in to remove it. This subgroup has much better outcomes than the two thirds who still have residual disease after neoadjuvant chemo.” The study was presented at the San Antonio Breast Cancer Symposium held December 10 - 14, 2019, in San Antonio, TX, USA.
Related Links:
Indiana University School of Medicine
Foundation Medicine
Latest Pathology News
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
- First-Of-Its-Kind Test Identifies Autism Risk at Birth
- AI Algorithms Improve Genetic Mutation Detection in Cancer Diagnostics
- Skin Biopsy Offers New Diagnostic Method for Neurodegenerative Diseases
- Fast Label-Free Method Identifies Aggressive Cancer Cells
- New X-Ray Method Promises Advances in Histology
- Single-Cell Profiling Technique Could Guide Early Cancer Detection
- Intraoperative Tumor Histology to Improve Cancer Surgeries
- Rapid Stool Test Could Help Pinpoint IBD Diagnosis
- AI-Powered Label-Free Optical Imaging Accurately Identifies Thyroid Cancer During Surgery
- Deep Learning–Based Method Improves Cancer Diagnosis
- ADLM Updates Expert Guidance on Urine Drug Testing for Patients in Emergency Departments
- New Age-Based Blood Test Thresholds to Catch Ovarian Cancer Earlier
- Genetics and AI Improve Diagnosis of Aortic Stenosis
- AI Tool Simultaneously Identifies Genetic Mutations and Disease Type
- Rapid Low-Cost Tests Can Prevent Child Deaths from Contaminated Medicinal Syrups
Channels
Clinical Chemistry
view channel
New PSA-Based Prognostic Model Improves Prostate Cancer Risk Assessment
Prostate cancer is the second-leading cause of cancer death among American men, and about one in eight will be diagnosed in their lifetime. Screening relies on blood levels of prostate-specific antigen... Read more
Extracellular Vesicles Linked to Heart Failure Risk in CKD Patients
Chronic kidney disease (CKD) affects more than 1 in 7 Americans and is strongly associated with cardiovascular complications, which account for more than half of deaths among people with CKD.... Read moreMolecular Diagnostics
view channel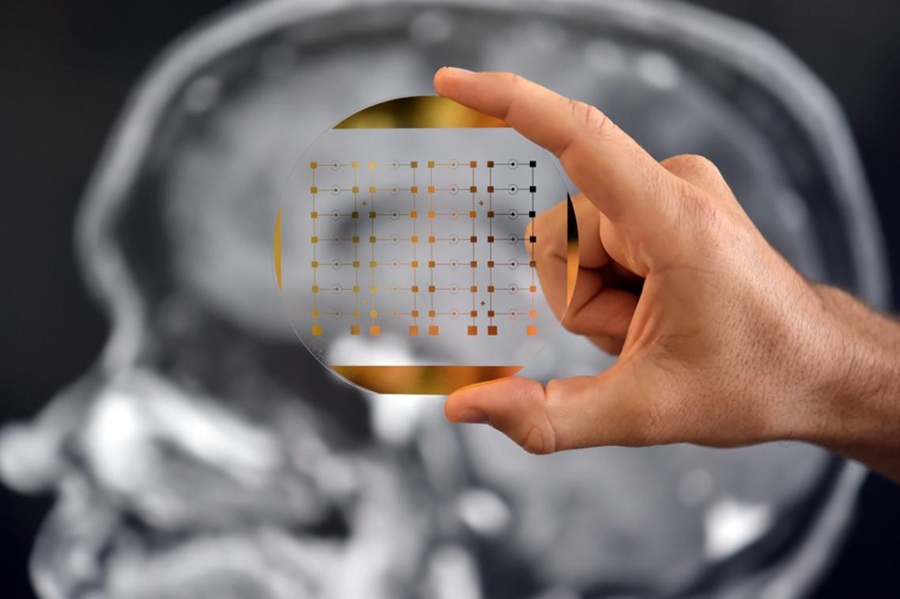
Diagnostic Device Predicts Treatment Response for Brain Tumors Via Blood Test
Glioblastoma is one of the deadliest forms of brain cancer, largely because doctors have no reliable way to determine whether treatments are working in real time. Assessing therapeutic response currently... Read more
Blood Test Detects Early-Stage Cancers by Measuring Epigenetic Instability
Early-stage cancers are notoriously difficult to detect because molecular changes are subtle and often missed by existing screening tools. Many liquid biopsies rely on measuring absolute DNA methylation... Read more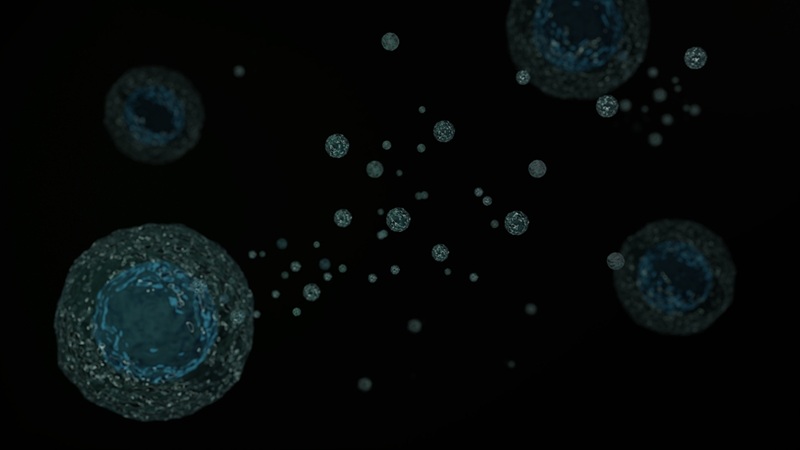
“Lab-On-A-Disc” Device Paves Way for More Automated Liquid Biopsies
Extracellular vesicles (EVs) are tiny particles released by cells into the bloodstream that carry molecular information about a cell’s condition, including whether it is cancerous. However, EVs are highly... Read more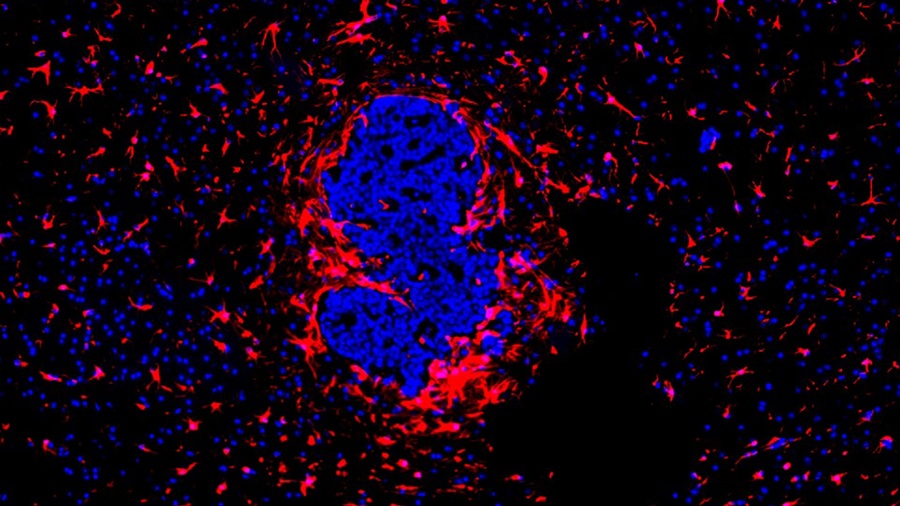
Blood Test Identifies Inflammatory Breast Cancer Patients at Increased Risk of Brain Metastasis
Brain metastasis is a frequent and devastating complication in patients with inflammatory breast cancer, an aggressive subtype with limited treatment options. Despite its high incidence, the biological... Read moreHematology
view channel
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read more
Fast and Easy Test Could Revolutionize Blood Transfusions
Blood transfusions are a cornerstone of modern medicine, yet red blood cells can deteriorate quietly while sitting in cold storage for weeks. Although blood units have a fixed expiration date, cells from... Read more
Automated Hemostasis System Helps Labs of All Sizes Optimize Workflow
High-volume hemostasis sections must sustain rapid turnaround while managing reruns and reflex testing. Manual tube handling and preanalytical checks can strain staff time and increase opportunities for error.... Read more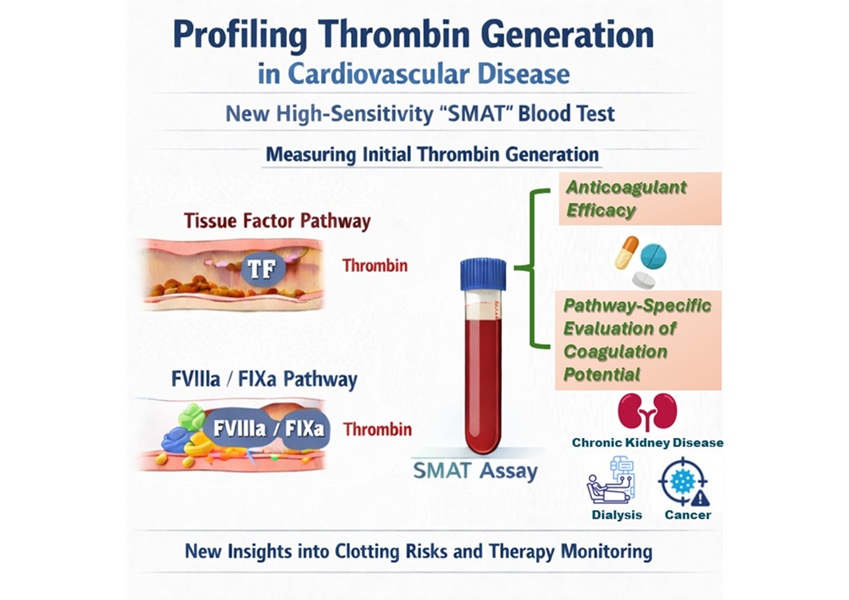
High-Sensitivity Blood Test Improves Assessment of Clotting Risk in Heart Disease Patients
Blood clotting is essential for preventing bleeding, but even small imbalances can lead to serious conditions such as thrombosis or dangerous hemorrhage. In cardiovascular disease, clinicians often struggle... Read moreImmunology
view channelBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read moreAI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
Infections caused by the pathogenic yeast Candida auris pose a significant threat to hospitalized patients, particularly those with weakened immune systems or those who have invasive medical devices.... Read moreTechnology
view channel
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreAptamer Biosensor Technology to Transform Virus Detection
Rapid and reliable virus detection is essential for controlling outbreaks, from seasonal influenza to global pandemics such as COVID-19. Conventional diagnostic methods, including cell culture, antigen... Read more
AI Models Could Predict Pre-Eclampsia and Anemia Earlier Using Routine Blood Tests
Pre-eclampsia and anemia are major contributors to maternal and child mortality worldwide, together accounting for more than half a million deaths each year and leaving millions with long-term health complications.... Read moreIndustry
view channelNew Collaboration Brings Automated Mass Spectrometry to Routine Laboratory Testing
Mass spectrometry is a powerful analytical technique that identifies and quantifies molecules based on their mass and electrical charge. Its high selectivity, sensitivity, and accuracy make it indispensable... Read more
AI-Powered Cervical Cancer Test Set for Major Rollout in Latin America
Noul Co., a Korean company specializing in AI-based blood and cancer diagnostics, announced it will supply its intelligence (AI)-based miLab CER cervical cancer diagnostic solution to Mexico under a multi‑year... Read more
Diasorin and Fisher Scientific Enter into US Distribution Agreement for Molecular POC Platform
Diasorin (Saluggia, Italy) has entered into an exclusive distribution agreement with Fisher Scientific, part of Thermo Fisher Scientific (Waltham, MA, USA), for the LIAISON NES molecular point-of-care... Read more