Immunoassays Compared for Diagnosis of Acute Murine Typhus Infections
|
By LabMedica International staff writers Posted on 10 Sep 2019 |
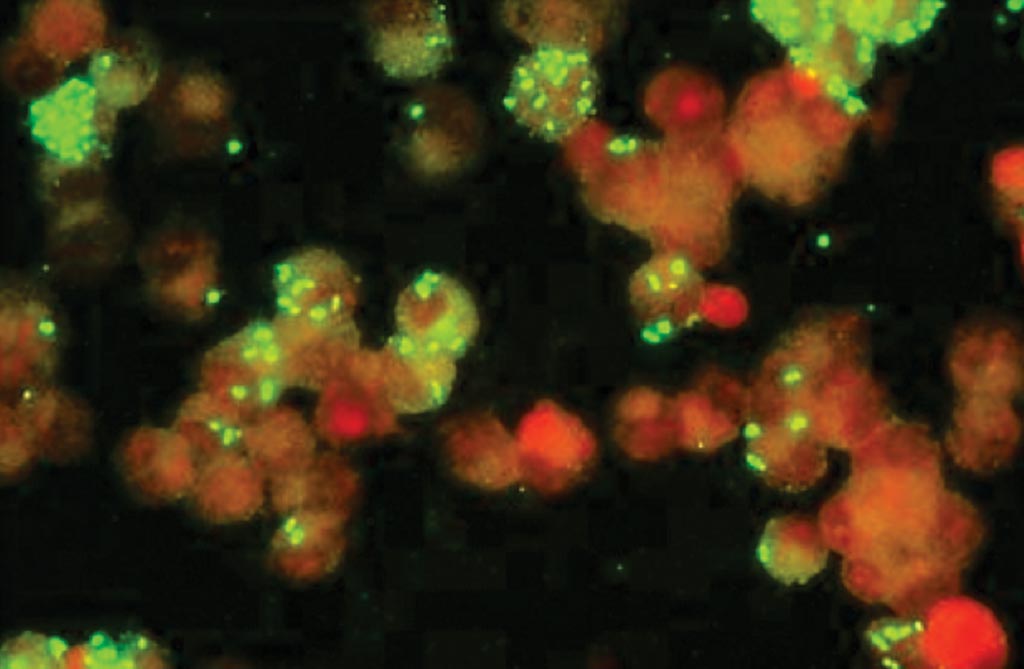
Image: A photomicrograph of an immunofluorescence assay (IFA) for Rickettsia (Photo courtesy of Fuller Laboratories).
Murine typhus is a disease transmitted by fleas and is caused by intracellular gram-negative bacteria called Rickettsia typhi, and manifested clinically with acute fever, chills, muscle pain, nausea, vomiting, stomach pain, cough and rash.
Appropriate rapid diagnostics are needed to distinguish it from other infections, as patient management varies. Due to low rickettsemia during acute illness, the sensitivity of real-time polymerase chain reaction (PCR) is highly variable. Thus, sero-diagnosis using immunofluorescence assay (IFA) remains the gold standard.
Scientists working with the Indonesia Research Partnership on Infectious Disease (Jakarta, Indonesia) obtained samples from eight government referral teaching hospitals in seven provincial capitals. Paired acute and convalescent plasma samples from 40 cases with confirmed R. typhi and 58 controls with another confirmed infection were used to evaluate the performance of commercial IgM and IgG enzyme-linked immunosorbent assay (ELISA) and IFA. The 58 paired plasma specimens that they used for controls were negative for R. typhi and Rickettsia spp., but positive for other pathogens by culture or molecular testing.
The immunofluorescence assay (IFA) was performed using kits from Focus Diagnostics (Cypress, CA, USA). The dilution for study samples was 1:64, and for provided positive controls was 1:32. Acute and convalescent specimens from each subject were performed simultaneously. Acute and convalescent plasma were tested simultaneously using ELISA kits from Fuller Laboratories (Fullerton, CA, USA). Microwells were coated with the outer surface membrane protein rOmp B purified from R. typhi. Optical density (OD) was measured at a wavelength of 450 nm.
The team reported that sensitivity and specificity of combined ELISA IgM and IgG anti-R. typhi using paired specimens were excellent (95.0% and 98.3%, respectively), comparable to combined IFA IgM and IgG (97.5% and 100%, respectively); sensitivity of ELISA IgM from acute specimens only was poor (45.0%), but specificity was excellent (98.3%). IFA IgM was more sensitive (77.5%), but less specific (89.7%) for single specimens. IgM was detected as early as day three of fever by ELISA and day four by IFA. Starting from day nine of illness, IgM was detected in all cases by IFA, while ELISA missed two specimens (days 10 and 25).
The authors concluded that their data supports the validity of ELISA in the diagnosis of R. typhi infection. As the specificity in acute specimens as well as sensitivity and specificity in convalescent specimens and paired specimens were excellent, ELISA is recommended when fluorescence microscopy is not feasible. However, IFA remains the method of choice if resources are available. ELISA is appropriate for resource-limited settings as it is easy to read, is objective, and has a high throughput. The study was published on August 26, 2019, in the journal Vector-Borne and Zoonotic Diseases.
Related Links:
Indonesia Research Partnership on Infectious Disease
Focus Diagnostics
Fuller Laboratories
Appropriate rapid diagnostics are needed to distinguish it from other infections, as patient management varies. Due to low rickettsemia during acute illness, the sensitivity of real-time polymerase chain reaction (PCR) is highly variable. Thus, sero-diagnosis using immunofluorescence assay (IFA) remains the gold standard.
Scientists working with the Indonesia Research Partnership on Infectious Disease (Jakarta, Indonesia) obtained samples from eight government referral teaching hospitals in seven provincial capitals. Paired acute and convalescent plasma samples from 40 cases with confirmed R. typhi and 58 controls with another confirmed infection were used to evaluate the performance of commercial IgM and IgG enzyme-linked immunosorbent assay (ELISA) and IFA. The 58 paired plasma specimens that they used for controls were negative for R. typhi and Rickettsia spp., but positive for other pathogens by culture or molecular testing.
The immunofluorescence assay (IFA) was performed using kits from Focus Diagnostics (Cypress, CA, USA). The dilution for study samples was 1:64, and for provided positive controls was 1:32. Acute and convalescent specimens from each subject were performed simultaneously. Acute and convalescent plasma were tested simultaneously using ELISA kits from Fuller Laboratories (Fullerton, CA, USA). Microwells were coated with the outer surface membrane protein rOmp B purified from R. typhi. Optical density (OD) was measured at a wavelength of 450 nm.
The team reported that sensitivity and specificity of combined ELISA IgM and IgG anti-R. typhi using paired specimens were excellent (95.0% and 98.3%, respectively), comparable to combined IFA IgM and IgG (97.5% and 100%, respectively); sensitivity of ELISA IgM from acute specimens only was poor (45.0%), but specificity was excellent (98.3%). IFA IgM was more sensitive (77.5%), but less specific (89.7%) for single specimens. IgM was detected as early as day three of fever by ELISA and day four by IFA. Starting from day nine of illness, IgM was detected in all cases by IFA, while ELISA missed two specimens (days 10 and 25).
The authors concluded that their data supports the validity of ELISA in the diagnosis of R. typhi infection. As the specificity in acute specimens as well as sensitivity and specificity in convalescent specimens and paired specimens were excellent, ELISA is recommended when fluorescence microscopy is not feasible. However, IFA remains the method of choice if resources are available. ELISA is appropriate for resource-limited settings as it is easy to read, is objective, and has a high throughput. The study was published on August 26, 2019, in the journal Vector-Borne and Zoonotic Diseases.
Related Links:
Indonesia Research Partnership on Infectious Disease
Focus Diagnostics
Fuller Laboratories
Latest Microbiology News
- Rapid Sequencing Could Transform Tuberculosis Care
- Blood-Based Viral Signature Identified in Crohn’s Disease
- Hidden Gut Viruses Linked to Colorectal Cancer Risk
- Three-Test Panel Launched for Detection of Liver Fluke Infections
- Rapid Test Promises Faster Answers for Drug-Resistant Infections
- CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
- Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
- AI-Powered Platform Enables Rapid Detection of Drug-Resistant C. Auris Pathogens
- New Test Measures How Effectively Antibiotics Kill Bacteria
- New Antimicrobial Stewardship Standards for TB Care to Optimize Diagnostics
- New UTI Diagnosis Method Delivers Antibiotic Resistance Results 24 Hours Earlier
- Breakthroughs in Microbial Analysis to Enhance Disease Prediction
- Blood-Based Diagnostic Method Could Identify Pediatric LRTIs
- Rapid Diagnostic Test Matches Gold Standard for Sepsis Detection
- Rapid POC Tuberculosis Test Provides Results Within 15 Minutes
- Rapid Assay Identifies Bloodstream Infection Pathogens Directly from Patient Samples
Channels
Clinical Chemistry
view channelNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read more
Electronic Nose Smells Early Signs of Ovarian Cancer in Blood
Ovarian cancer is often diagnosed at a late stage because its symptoms are vague and resemble those of more common conditions. Unlike breast cancer, there is currently no reliable screening method, and... Read moreMolecular Diagnostics
view channel
Blood Test Could Help Guide Treatment Decisions in Germ Cell Tumors
Chemotherapy is often highly effective for germ cell tumors, but in a subset of patients, the disease does not respond well to standard treatment. For these individuals, doctors may consider high-dose... Read more
Blood Test Could Spot Common Post-Surgery Condition Early
Heterotopic ossification (HO), the abnormal formation of bone in soft tissue, is a common complication following hip replacement surgery. The condition affects nearly one in three patients and can lead... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreMicrobiology
view channel
Rapid Sequencing Could Transform Tuberculosis Care
Tuberculosis remains the world’s leading cause of death from a single infectious agent, responsible for more than one million deaths each year. Diagnosing and monitoring the disease can be slow because... Read more
Blood-Based Viral Signature Identified in Crohn’s Disease
Crohn’s disease is a chronic inflammatory intestinal disorder affecting approximately 0.4% of the European population, with symptoms and progression that vary widely. Although viral components of the microbiome... Read morePathology
view channel
World’s First Optical Microneedle Device to Enable Blood-Sampling-Free Clinical Testing
Blood sampling is one of the most common clinical procedures, but it can be difficult or uncomfortable for many patients, especially older adults or individuals with certain medical conditions.... Read more
Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
Polymerase Chain Reaction (PCR) testing became widely recognized during the COVID-19 pandemic as a powerful method for detecting viruses such as SARS-CoV-2. PCR belongs to a group of diagnostic methods... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more






















