Establishment of Biomarker Panel May Lead to Rapid Tests for Early Diagnosis of Pancreatic Cancer
|
By LabMedica International staff writers Posted on 10 Apr 2016 |
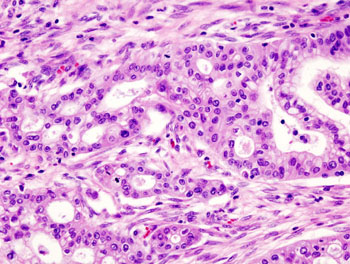
Image: Micrograph of pancreatic ductal adenocarcinoma (the most common type of pancreatic cancer) (Photo courtesy of Wikimedia Commons).
A panel comprising five genetic biomarkers was shown to accurately differentiate among tissues from pancreatic tumors and those taken from various non-malignant sources.
Investigators at Beth Israel Deaconess Medical Center (Boston, MA, USA) applied innovative data normalization and gene selection approaches to analyze a number of publicly available pancreatic cancer gene expression datasets. They combined the statistical power of multiple genomic studies while masking their variability and batch effects to identify robust early diagnostic biomarkers of pancreatic cancer.
The investigators established a panel comprising the genes TMPRSS4 (Transmembrane protease, serine 4), AHNAK2 (AHNAK nucleoprotein 2), POSTN (Periostin), ECT2 (Epithelial cell transforming 2), and SERPINB5 (Serpin peptidase inhibitor, clade B (ovalbumin), member 5) that achieved on average 95% sensitivity and 89% specificity in discriminating pancreatic ductal adenocarcinoma (PDAC) from non-tumor samples in four training sets and similar performance in five independent validation datasets. The five-gene classifier accurately discriminated PDAC from chronic pancreatitis, other cancers, and non-tumor samples from PDAC precursors in three independent datasets.
PDAC-specific expression of the biomarker panel was measured by qRT-PCR (qualitative real-time PCR) in microdissected patient-derived FFPE (formalin-fixed, paraffin-embedded) tissues. Cell-based assays were then used to assess the impact of two of the biomarkers, TMPRSS4 and ECT2, on PDAC cells.
Results revealed that knock-down of TMPRSS4 and ECT2 reduced PDAC soft agar growth and cell viability, and TMPRSS4 knockdown also blocked PDAC migration and invasion.
“Pancreatic cancer is a devastating disease with a death rate close to the incidence rate,” said senior author Dr. Towia Libermann, professor of medicine at Beth Israel Deaconess Medical Center. “Because more than 90% of pancreatic cancer cases are diagnosed at the metastatic stage, when there are only limited therapeutic options, earlier diagnosis is anticipated to have a major impact on extending life expectancy for patients. There has been a lack of reliable markers, early indicators, and risk factors associated with pancreatic cancer, but this new way of differentiating between healthy and malignant tissue offers hope for earlier diagnosis and treatment.”
“Because these five genes are turned on so early in the development of pancreatic cancer, they may play roles as drivers of this disease and may be exciting targets for therapies,” said Dr. Libermann. “Moving forward, we will explore the potential to convert this tissue-based diagnostic into a noninvasive blood or urine test.”
The study was published in the March 16, 2016, online edition of the journal Oncotarget.
Related Links:
Beth Israel Deaconess Medical Center
Investigators at Beth Israel Deaconess Medical Center (Boston, MA, USA) applied innovative data normalization and gene selection approaches to analyze a number of publicly available pancreatic cancer gene expression datasets. They combined the statistical power of multiple genomic studies while masking their variability and batch effects to identify robust early diagnostic biomarkers of pancreatic cancer.
The investigators established a panel comprising the genes TMPRSS4 (Transmembrane protease, serine 4), AHNAK2 (AHNAK nucleoprotein 2), POSTN (Periostin), ECT2 (Epithelial cell transforming 2), and SERPINB5 (Serpin peptidase inhibitor, clade B (ovalbumin), member 5) that achieved on average 95% sensitivity and 89% specificity in discriminating pancreatic ductal adenocarcinoma (PDAC) from non-tumor samples in four training sets and similar performance in five independent validation datasets. The five-gene classifier accurately discriminated PDAC from chronic pancreatitis, other cancers, and non-tumor samples from PDAC precursors in three independent datasets.
PDAC-specific expression of the biomarker panel was measured by qRT-PCR (qualitative real-time PCR) in microdissected patient-derived FFPE (formalin-fixed, paraffin-embedded) tissues. Cell-based assays were then used to assess the impact of two of the biomarkers, TMPRSS4 and ECT2, on PDAC cells.
Results revealed that knock-down of TMPRSS4 and ECT2 reduced PDAC soft agar growth and cell viability, and TMPRSS4 knockdown also blocked PDAC migration and invasion.
“Pancreatic cancer is a devastating disease with a death rate close to the incidence rate,” said senior author Dr. Towia Libermann, professor of medicine at Beth Israel Deaconess Medical Center. “Because more than 90% of pancreatic cancer cases are diagnosed at the metastatic stage, when there are only limited therapeutic options, earlier diagnosis is anticipated to have a major impact on extending life expectancy for patients. There has been a lack of reliable markers, early indicators, and risk factors associated with pancreatic cancer, but this new way of differentiating between healthy and malignant tissue offers hope for earlier diagnosis and treatment.”
“Because these five genes are turned on so early in the development of pancreatic cancer, they may play roles as drivers of this disease and may be exciting targets for therapies,” said Dr. Libermann. “Moving forward, we will explore the potential to convert this tissue-based diagnostic into a noninvasive blood or urine test.”
The study was published in the March 16, 2016, online edition of the journal Oncotarget.
Related Links:
Beth Israel Deaconess Medical Center
Latest Pathology News
- AI Tool Predicts Chemotherapy Response from Biopsy Slides
- Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
- World’s First Optical Microneedle Device to Enable Blood-Sampling-Free Clinical Testing
- Novel mcPCR Technology to Transform Testing of Clinical Samples
- Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
- Molecular Imaging to Reduce Need for Melanoma Biopsies
- Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
- AI-Powered 3D Scanning System Speeds Cancer Screening
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Channels
Clinical Chemistry
view channel
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreNew Blood Test Index Offers Earlier Detection of Liver Scarring
Metabolic fatty liver disease is highly prevalent and often silent, yet it can progress to fibrosis, cirrhosis, and liver failure. Current first-line blood test scores frequently return indeterminate results,... Read moreMolecular Diagnostics
view channel
World’s First Portable POC Test Simultaneously Detects Four Common STIs in One Hour
Sexually transmitted infections (STIs) often present with similar symptoms, making accurate diagnosis challenging without laboratory testing. Delays in identifying the exact infection can lead to inappropriate... Read more
Simple One-Hour Saliva Test Detects Common Cancers
Early detection is critical for improving cancer outcomes, yet many diagnostic tests rely on invasive procedures such as blood draws or biopsies. Researchers are exploring simpler approaches that could... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
Tuberculosis (TB) remains one of the world’s leading infectious disease killers, yet millions of cases go undiagnosed or are detected too late. Barriers such as reliance on sputum samples, limited laboratory... Read more
New Imaging Approach Could Help Predict Dangerous Gut Infection
Clostridioides difficile infections affect roughly half a million people in the United States each year and are a leading cause of infectious diarrhea in healthcare settings. The bacterium can trigger... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more





















