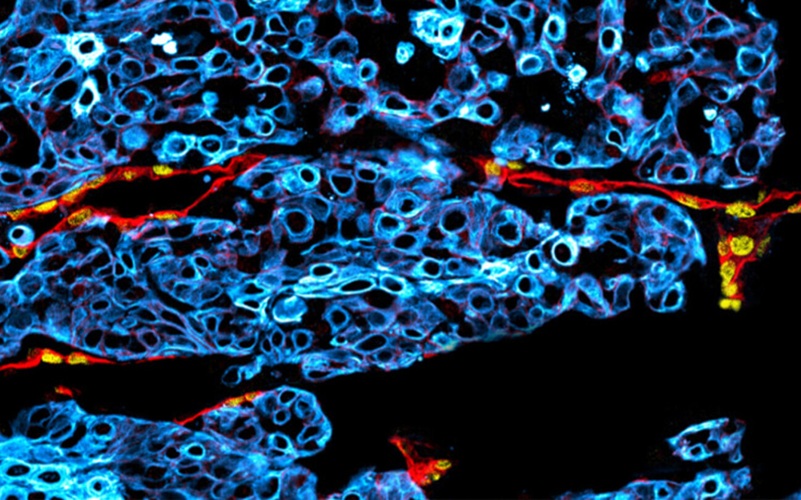
DNA Hypermethylation Assay Confirms Negative Prostate Cancer Biopsy Results
|
By LabMedica International staff writers Posted on 10 Jun 2014 |
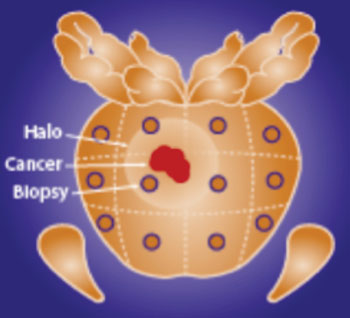
Image: ConfirmMDx detects an epigenetic field effect or “halo” associated with the cancerization process at the DNA level in cells adjacent to cancer foci. This epigenetic “halo” around a cancer lesion can be present despite having a normal appearance under the microscope (Photo courtesy of MDxHealth).
A commercially available assay that measures the level of hypermethylated DNA in tissue samples was found to accurately identify negative-for-cancer prostate tissues in more than 88% of cases.
Approximately 700,000 men in the USA receive a negative prostate biopsy result; however approximately 25% of these results are false-negative. Under the current standard of care, prostate biopsy procedures collect 10–12 needle biopsy cores on average, effectively sampling less than 1% of a man’s prostate. This approach leaves men at risk of occult cancer, leading to a high rate of repeat biopsies, often on cancer-free men. The MDxHealth (Herstal, Belgium) "ConfirmMDx for Prostate Cancer" assay addresses the unmet medical need for a clinically effective diagnostic test to address this dilemma. "ConfirmMDx for Prostate Cancer" is an epigenetic assay to help distinguish patients who have a true-negative biopsy from those who may have occult cancer. The test helps urologists rule-out prostate cancer-free men from undergoing unnecessary repeat biopsies and, helps rule-in high risk patients who may require repeat biopsies and potential treatment.
In a study to validate the use of the MDxHealth assay, investigators at Johns Hopkins University (Baltimore, MD, USA) evaluated archived negative-for-cancer prostate biopsy core tissue samples from 350 subjects from five urologic centers in the USA. All subjects underwent a repeat biopsy within 24 months with a negative (controls) or positive (cases) histopathological result. The MDxHealth assay profiled methylation levels for the known tumor suppressor genes GSTP1, APC, and RASSF1, which are silenced by hypermethylation and fail to block cancer development.
Results of analysis of the two biopsy specimens from each patient showed that average levels of APC and RASSF1 were about twice as high in the 92 subjects whose second biopsies yielded positive results, as compared to the 228 with two negative biopsies. For GSTP1, the levels were more than eight times higher in the cancerous biopsies.
“Overall, if there is an absence of methylation in all three biomarkers, there is an 88% likelihood you do not have cancer,” said senior author Dr. Jonathan Epstein, professor of pathology, urology, and oncology at Johns Hopkins University. “The test is not 100% of an assurance, but it is a major step forward.”
“Often, one biopsy is not enough to definitively rule out prostate cancer,” said Dr. Epstein. “Our research finds that by looking for the presence or absence of cancer in a different way, we may be able to offer many men peace of mind without putting them through the pain, bleeding and risk of infection that can come with a repeat biopsy. It turns out as many as 20% of men have prostate cancer, even if their first biopsy results are negative. Approximately 40% of men with a negative biopsy go on to receive a second biopsy. Many high-risk men fear sampling errors in their initial biopsy, which often leads to a high rate of follow-up procedures to merely confirm the absence of the disease.”
The study was published in the April 16, 2014, online edition of the Journal of Urology.
Related Links:
MDxHealth
Johns Hopkins University
Approximately 700,000 men in the USA receive a negative prostate biopsy result; however approximately 25% of these results are false-negative. Under the current standard of care, prostate biopsy procedures collect 10–12 needle biopsy cores on average, effectively sampling less than 1% of a man’s prostate. This approach leaves men at risk of occult cancer, leading to a high rate of repeat biopsies, often on cancer-free men. The MDxHealth (Herstal, Belgium) "ConfirmMDx for Prostate Cancer" assay addresses the unmet medical need for a clinically effective diagnostic test to address this dilemma. "ConfirmMDx for Prostate Cancer" is an epigenetic assay to help distinguish patients who have a true-negative biopsy from those who may have occult cancer. The test helps urologists rule-out prostate cancer-free men from undergoing unnecessary repeat biopsies and, helps rule-in high risk patients who may require repeat biopsies and potential treatment.
In a study to validate the use of the MDxHealth assay, investigators at Johns Hopkins University (Baltimore, MD, USA) evaluated archived negative-for-cancer prostate biopsy core tissue samples from 350 subjects from five urologic centers in the USA. All subjects underwent a repeat biopsy within 24 months with a negative (controls) or positive (cases) histopathological result. The MDxHealth assay profiled methylation levels for the known tumor suppressor genes GSTP1, APC, and RASSF1, which are silenced by hypermethylation and fail to block cancer development.
Results of analysis of the two biopsy specimens from each patient showed that average levels of APC and RASSF1 were about twice as high in the 92 subjects whose second biopsies yielded positive results, as compared to the 228 with two negative biopsies. For GSTP1, the levels were more than eight times higher in the cancerous biopsies.
“Overall, if there is an absence of methylation in all three biomarkers, there is an 88% likelihood you do not have cancer,” said senior author Dr. Jonathan Epstein, professor of pathology, urology, and oncology at Johns Hopkins University. “The test is not 100% of an assurance, but it is a major step forward.”
“Often, one biopsy is not enough to definitively rule out prostate cancer,” said Dr. Epstein. “Our research finds that by looking for the presence or absence of cancer in a different way, we may be able to offer many men peace of mind without putting them through the pain, bleeding and risk of infection that can come with a repeat biopsy. It turns out as many as 20% of men have prostate cancer, even if their first biopsy results are negative. Approximately 40% of men with a negative biopsy go on to receive a second biopsy. Many high-risk men fear sampling errors in their initial biopsy, which often leads to a high rate of follow-up procedures to merely confirm the absence of the disease.”
The study was published in the April 16, 2014, online edition of the Journal of Urology.
Related Links:
MDxHealth
Johns Hopkins University
Latest Pathology News
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
- First-Of-Its-Kind Test Identifies Autism Risk at Birth
- AI Algorithms Improve Genetic Mutation Detection in Cancer Diagnostics
- Skin Biopsy Offers New Diagnostic Method for Neurodegenerative Diseases
- Fast Label-Free Method Identifies Aggressive Cancer Cells
- New X-Ray Method Promises Advances in Histology
- Single-Cell Profiling Technique Could Guide Early Cancer Detection
- Intraoperative Tumor Histology to Improve Cancer Surgeries
- Rapid Stool Test Could Help Pinpoint IBD Diagnosis
Channels
Clinical Chemistry
view channel
Simple Blood Test Offers New Path to Alzheimer’s Assessment in Primary Care
Timely evaluation of cognitive symptoms in primary care is often limited by restricted access to specialized diagnostics and invasive confirmatory procedures. Clinicians need accessible tools to determine... Read more
Existing Hospital Analyzers Can Identify Fake Liquid Medical Products
Counterfeit and substandard medicines remain a serious global health threat, with World Health Organization estimates suggesting that 10.5% of medicines in low- and middle-income countries are either fake... Read moreMolecular Diagnostics
view channel
New Genome Sequencing Technique Measures Epstein-Barr Virus in Blood
The Epstein–Barr virus (EBV) infects up to 95% of adults worldwide and remains in the body for life. While usually kept under control, the virus is linked to cancers such as Hodgkin’s lymphoma and autoimmune... Read more
Blood Test Boosts Early Detection of Brain Cancer
Brain and central nervous system (CNS) tumors are often diagnosed at an advanced stage, when treatment options are limited, and survival rates remain low. Around 300,000 new cases are diagnosed each year... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read more
Whole-Genome Sequencing Approach Identifies Cancer Patients Benefitting From PARP-Inhibitor Treatment
Targeted cancer therapies such as PARP inhibitors can be highly effective, but only for patients whose tumors carry specific DNA repair defects. Identifying these patients accurately remains challenging,... Read more
Ultrasensitive Liquid Biopsy Demonstrates Efficacy in Predicting Immunotherapy Response
Immunotherapy has transformed cancer treatment, but only a small proportion of patients experience lasting benefit, with response rates often remaining between 10% and 20%. Clinicians currently lack reliable... Read moreMicrobiology
view channel
Three-Test Panel Launched for Detection of Liver Fluke Infections
Parasitic liver fluke infections remain endemic in parts of Asia, where transmission commonly occurs through consumption of raw freshwater fish or aquatic plants. Chronic infection is a well-established... Read more
Rapid Test Promises Faster Answers for Drug-Resistant Infections
Drug-resistant pathogens continue to pose a growing threat in healthcare facilities, where delayed detection can impede outbreak control and increase mortality. Candida auris is notoriously difficult to... Read more
CRISPR-Based Technology Neutralizes Antibiotic-Resistant Bacteria
Antibiotic resistance has accelerated into a global health crisis, with projections estimating more than 10 million deaths per year by 2050 as drug-resistant “superbugs” continue to spread.... Read more
Comprehensive Review Identifies Gut Microbiome Signatures Associated With Alzheimer’s Disease
Alzheimer’s disease affects approximately 6.7 million people in the United States and nearly 50 million worldwide, yet early cognitive decline remains difficult to characterize. Increasing evidence suggests... Read moreTechnology
view channel
Blood Test “Clocks” Predict Start of Alzheimer’s Symptoms
More than 7 million Americans live with Alzheimer’s disease, and related health and long-term care costs are projected to reach nearly USD 400 billion in 2025. The disease has no cure, and symptoms often... Read more
AI-Powered Biomarker Predicts Liver Cancer Risk
Liver cancer, or hepatocellular carcinoma, causes more than 800,000 deaths worldwide each year and often goes undetected until late stages. Even after treatment, recurrence rates reach 70% to 80%, contributing... Read more
Robotic Technology Unveiled for Automated Diagnostic Blood Draws
Routine diagnostic blood collection is a high‑volume task that can strain staffing and introduce human‑dependent variability, with downstream implications for sample quality and patient experience.... Read more
ADLM Launches First-of-Its-Kind Data Science Program for Laboratory Medicine Professionals
Clinical laboratories generate billions of test results each year, creating a treasure trove of data with the potential to support more personalized testing, improve operational efficiency, and enhance patient care.... Read moreIndustry
view channel
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more








 (3) (1).png)