Sensitive Assays Enable Early Detection of Prion Infection
By LabMedica International staff writers
Posted on 04 Feb 2019
Researchers working with rodent models have demonstrated the potential for developing a skin test for the early diagnosis of prion diseases in humans.Posted on 04 Feb 2019
Prions are proteinaceous, infectious particles that completely lack any genetic material. These particles are transmissible pathogens, which cause neurodegenerative disorders in humans and animals. Prions show strikingly different biochemical and biophysical properties from other pathogens, such as fungi, bacteria, and viruses, as well as differing host-pathogen interactions. Prions are unusually resistant to many conventional chemical and physical treatments to reduce infectivity, such as intensive ultraviolet radiation, heat, and nuclease treatment.
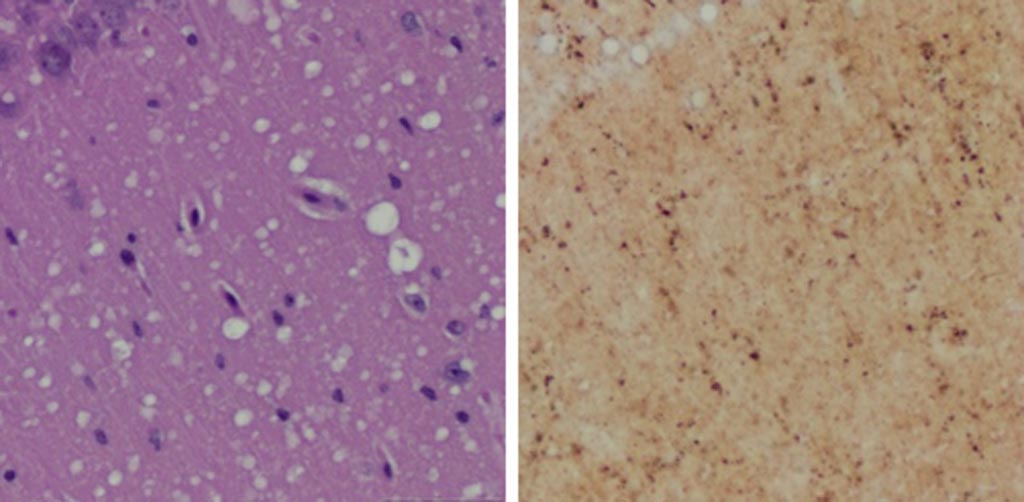
Image: A Microscopic examination of brain tissues of prion-infected animals. (Left) Staining shows spongiform degeneration. (Right) Staining shows intense misfolded prion protein (Photo courtesy of Case Western Reserve University).
Furthermore, prion infection induces no humoral or innate immune responses in the host. Prions are also peculiar in the way they multiply, which involves protein-protein interactions followed by conformational conversion. The normal form of prion protein is called PrPC, while the infectious form is called PrPSc – the C refers to cellular PrP (prion protein), while the Sc refers to scrapie, the prototypic prion disease, occurring in sheep. While PrPC is structurally well defined, PrPSc is polydisperse and impossible to define.
A definitive pre-mortem diagnosis of a prion disease, such as Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle, or chronic wasting disease (CWD) in elk and deer depends on brain biopsy for prion detection, and no validated alternative preclinical diagnostic tests have been reported to date.
To improve this situation, investigators at Case Western Reserve University (Cleveland, OH, USA) sought to determine the feasibility of using a noninvasive skin test for preclinical diagnosis. This idea was based on previous findings, which showed that autopsy skin samples from human prion disease patients exhibited prion seeding and infectivity.
To test the hypothesis, the investigators examined skin PrPSc in hamsters and humanized transgenic (Tg) mice at different time points after intracerebral prion inoculation using the highly sensitive RT-QuIC and sPMCA assays.
The real-time quaking induced conversion (RT-QuIC) assay uses recombinant prion protein to which potentially infectious tissue homogenate is added. If the tissue has prion seeding activity, it induces aggregation in recombinant protein, which can be monitored by the fluorophore Thioflavin T (ThT). For aggregation to occur, intermittent double-orbital shaking at 42 degrees Celsius is required over the assay duration of up to 68 hours. ThT fluorescence is acquired every 15 minutes to report on aggregation status.
The serial protein misfolding cyclic amplification (sPMCA) technique initially incubates a small amount of abnormal prion with an excess of normal protein, so that some conversion takes place. The growing chain of misfolded protein is then blasted with ultrasound, breaking it down into smaller chains and so rapidly increasing the amount of abnormal protein available to cause conversions. By repeating the cycle, the mass of normal protein is rapidly changed into misfolded PrPSc prions. One round of PMCA cycling results in a 2500-fold increase in sensitivity of detection over western blotting, whereas two and seven rounds of successive PMCA cycling result in six million- and three billion-fold increases in sensitivity of detection over western blotting. Thus, PMCA is capable of detecting as little as a single molecule of oligomeric infectious PrPSc.
The investigators reported in the January 16, 2019, online edition of the journal Nature Communications that sPMCA detected skin PrPSc as early as two weeks post inoculation (wpi) in hamsters and four wpi in Tg40h mice. The RT-QuIC assay revealed earliest skin prion-seeding activity at three wpi in hamsters and 20 wpi in Tg40h mice. Unlike prion-inoculated animals, mock-inoculated animals showed detectable skin/brain PrPSc only after long cohabitation periods with scrapie-infected animals.
“Currently a definitive diagnosis of Creutzfeldt-Jakob disease is dependent on the examination of diseased brain tissue obtained at biopsy or autopsy. It has been impossible to detect at the early preclinical stage,” said senior author Dr. Wenquan Zou, associate professor of pathology at Case Western Reserve University. “Since the skin is readily accessible and skin biopsy is minimally invasive, detection of skin prions will be very useful for monitoring disease progression and assessing therapeutic efficacy during clinical trials or treatments when prion therapy becomes available in the future.”
“Sensitive, minimally invasive detection of various misfolded proteins in skin, such as tau in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease, could be highly valuable for disease diagnosis and monitoring of disease progression and efficacy of treatments,” said Dr. Zou. “It is possible that the skin will ultimately serve as a mirror for us to monitor these misfolded proteins that accumulate and damage the brain in patients with these conditions.”
Related Links:
Case Western Reserve University













