Researchers Aim to Reduce Variability of Gene Therapy Vectors
By LabMedica International staff writers
Posted on 30 Oct 2018
A recently published study illuminated a previously unknown aspect of adeno-associated virus capsid heterogeneity and highlighted its importance in the development of these vectors for use in gene therapy applications.Posted on 30 Oct 2018
Adeno-associated virus (AAV) is a small virus that infects humans and some other primate species. AAV is not currently known to cause disease. The virus causes a very mild immune response, lending further support to its apparent lack of pathogenicity. In many cases, AAV vectors integrate into the host cell genome, which can be important for certain applications, but can also have unwanted consequences. Gene therapy vectors using AAV can infect both dividing and quiescent cells and persist in an extrachromosomal state without integrating into the genome of the host cell, although in the native virus some integration of virally carried genes into the host genome does occur. These features make AAV a very attractive candidate for creating viral vectors for gene therapy.
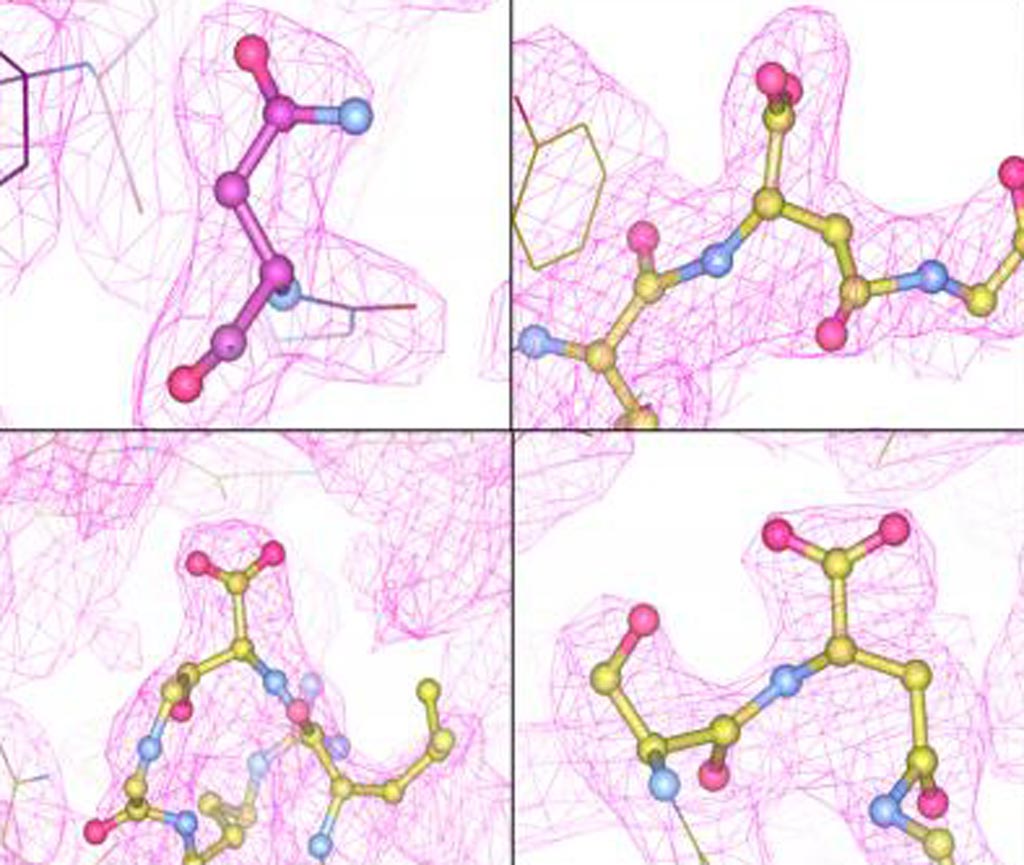
Image: A re-examination of adeno-associated virus capsid structural data revealed evidence for alterations at several sites (Photo courtesy of Lakshmanan Govindasamy, University of Pennsylvania).
Post-translational modification of the AAV capsids is a poorly understood factor in the development of these viral vectors into pharmaceutical products. In this regard, investigators at the University of Pennsylvania (Philadelphia, USA) reported the extensive capsid deamidation of adeno-associated virus serotype eight and seven other diverse adeno-associated virus serotypes, with supporting evidence from structural, biochemical, and mass spectrometry approaches.
The investigators reported in the October 18, 2018, online edition of the journal Molecular Therapy that the extent of deamidation at each site depended on the vector’s age and multiple primary-sequence and three-dimensional structural factors. However, the extent of deamidation was largely independent of the vector recovery and purification conditions.
The investigators also examined mutational strategies to stabilize side-chain amides, improving vector transduction and reducing the lot-to-lot molecular variability that presents a key concern in biologics manufacturing.
"This study determined how a viral vector can lose activity during harvest and purification in the manufacturing process," said senior author Dr. James Wilson, professor of medicine at the University of Pennsylvania. "That discovery led us to uncover new ways to prevent this from happening in order to deliver gene therapy treatments in a safer and more efficient way. Our discovery of these functionally important modifications reinforces the significance of improved analytical assays to characterize AAV products."
Related Links:
University of Pennsylvania













