Hydrogel-Based Model System Mimics Cellular Signaling Processes
By LabMedica International staff writers
Posted on 07 Nov 2017
An artificial hydrogel-based model system responds to chemical signals by binding or releasing bound proteins in a manner similar to processes occurring in living cells.Posted on 07 Nov 2017
A variety of hydrogels have been synthesized for controlling the release of signaling molecules in applications such as drug delivery and regenerative medicine. However, it remains challenging to synthesize hydrogels with the ability to control the release of signaling molecules sequentially or periodically under physiological conditions as living cells do in response to the variation of metabolism.
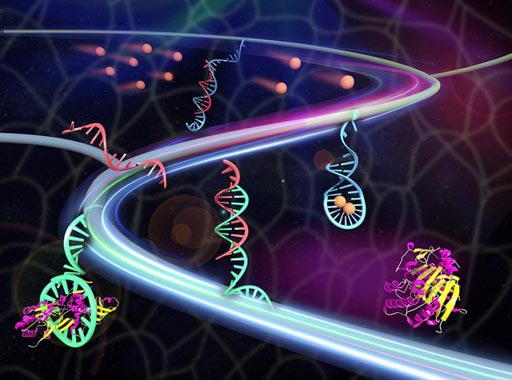
Image: A synthetic tissue releases therapeutic proteins (maroon/yellow) once triggered by metabolites (sandy brown). The metabolites contact with the double-stranded DNA (red/blue) to release the red triggering DNA. The triggering DNA activates the aptamer (cyan)-protein complex to release the protein (Photo courtesy of Xin Zou and Jinping Lai / Pennsylvania State University).
To meet this challenge, investigators at Pennsylvania State University (University Park, USA) prepared a novel hydrogel from polyethylene glycol that was infused with two different types of DNA. One was an aptamer, a short strand of DNA that bound the molecules to be released from the hydrogel. The other was a double-stranded helical molecule of DNA designed to react to the metabolic signal and initiate the chemical release process.
The investigators reported in the November 2017 issue of the journal Chemical Science that they had used adenosine as the low molecular weight signaling molecule and platelet-derived growth factor (PDGF) as the signaling protein to be released. The investigators analyzed the adenosine-PDGF hydrogel system and found that without the low molecular weight signal molecule, the amount of signaling protein released by the hydrogel was very small. When adenosine was added, the hydrogel released about 28% percent of the target PDG signaling protein. Other molecules similar to adenosine, such as guanosine and uridine did not cause the release of PDGF from the hydrogel.
"We have only done this recently in a petri dish," said senior author Dr. Yong Wang, professor of biomedical engineering at Pennsylvania State University. "We did tests using smooth muscle cells, but we would of course like to do this in a living animal. Eventually we would like to use this system for controlled drug delivery and other biological actions."
Related Links:
Pennsylvania State University













