Study Details Interaction between Galectin-1 and Carbohydrate Ligands
By LabMedica International staff writers
Posted on 23 Aug 2017
A team of German researchers used advanced X-ray crystallography techniques to develop a structural understanding of the interaction between the galectin-1 protein and its carbohydrate ligands.Posted on 23 Aug 2017
The galectins are a family of beta-galactoside-binding proteins implicated in modulating cell-cell and cell-matrix interactions. Galectins are defined by their binding specificity for beta-galactoside sugars, such as N-acetyllactosamine (Galbeta1-3GlcNAc or Galbeta1-4GlcNAc), which can be bound to proteins by either N-linked or O-linked glycosylation. Unlike the majority of lectins they are not membrane bound, but soluble proteins with both intra- and extracellular functions. They have distinct but overlapping distributions but are found primarily in the cytosol, nucleus, extracellular matrix, or in circulation. Galectin-1 may act as an autocrine negative growth factor that regulates cell proliferation. Expression of galectin-1in Hodgkin lymphoma has been shown to mediate immunosuppression of CD8+ T-cells.
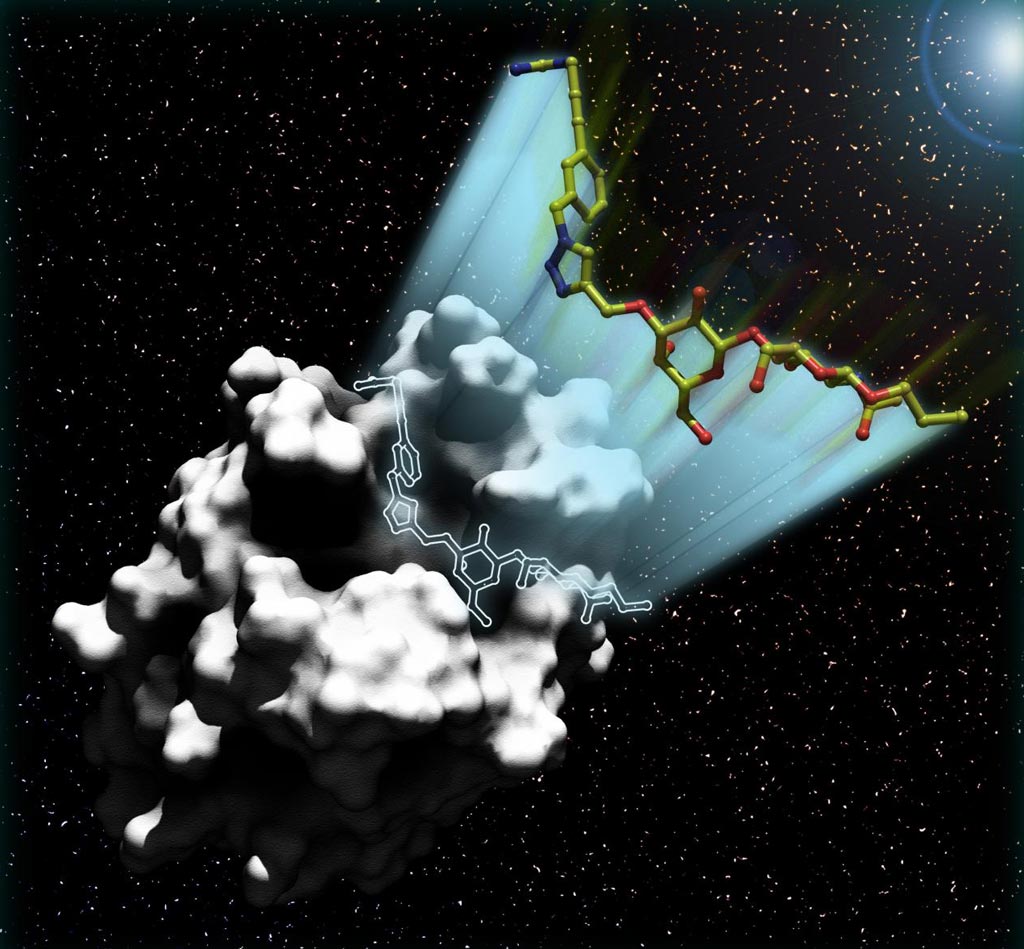
Image: In this model, the complex sugar molecule (colored) attaches to the tumor protein galectin-1, which is shown in black and white (Photo courtesy of Workgroup Seibel, VCH-Wiley).
In order to study the interaction between galectin-1 and its ligands, investigators at the University of Wurzburg (Germany) prepared a multifunctional natural scaffold based on N-acetyllactosamine (LacNAc). It served as an anchor motif for further expansion by the Sharpless–Huisgen–Meldal reaction, which resulted in ligands with a binding mode almost identical to that of the natural carbohydrate template. X-ray crystallography was then used to provide a structural understanding of the galectin-1–ligand interactions.
"We have equipped the sugar molecule with a docking site, for example, to connect it with a fluorescent dye or a drug," said senior author Dr. Jürgen Seibel, professor of organic chemistry at the University of Wurzburg. "Among other things, it is known that galectin-1 hides the tumor cells from the immune system. When galectin-1 is blocked, the immune system can recognize the tumor and attack it with T cells."
Results of the X-ray crystallography study were published in the August 4, 2017, issue of the journal ChemBioChem.
Related Links:
University of Wurzburg













