Shortened p53 Protein Promotes Tumor Growth by Modulating Mitochondrial Function
By LabMedica International staff writers
Posted on 20 Dec 2016
A shortened version of the p53 protein - caused by a mutation in the TP53 tumor suppressor gene – has been found to promote rather than impede tumor growth.Posted on 20 Dec 2016
The gene that encodes p53 is the most frequently mutated gene found in many types of cancer, and notably in most late-stage cancers. While most p53 gene mutations prevent p53 from being functional, investigators at Cold Spring Harbor Laboratory (NY, USA) discovered a variety of mutated p53 protein that actually promoted tumor growth.
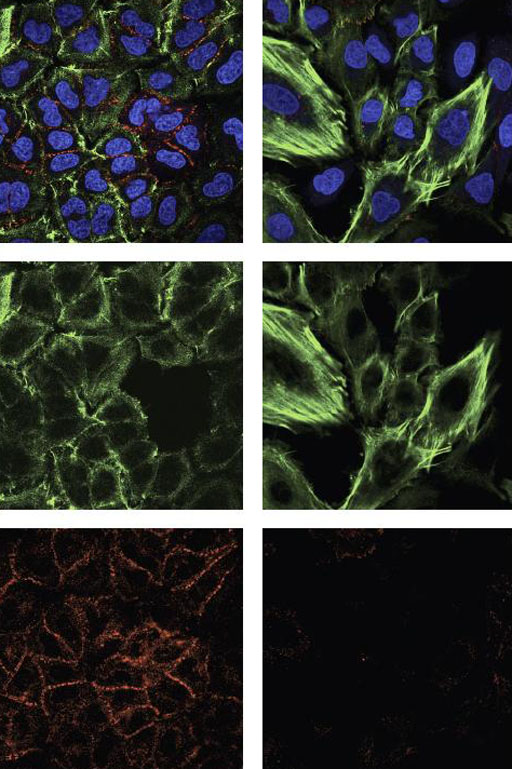
Image: Cells that express exon 6-truncated p53 protein exhibit structural features that reflect their reprogramming away from stability and toward proliferation and metastasis. This was apparent when comparing cells that do not express the truncated form of the protein (left column) with those that do (right column). The two images at the top are composites, with blue indicating DNA (i.e., cell nuclei); and green and red corresponding, respectively, with the proteins actin and e-cadherin. In the cells reprogrammed by truncated p53 proteins, actin fibers (middle image) show stress, while the signal from e-cadherin \"glue\" drops out altogether (bottom image). These cells are much more likely to break away from tissue and travel in the body (Photo courtesy of Sordella Laboratory, Cold Spring Harbor Laboratory).
The investigators reported in the October 19, 2016, online edition of the journal eLife that p53 proteins truncated after the sixth protein-coding segment (exon-6) no longer functioned as tumor suppressors but instead promoted cancer by directly altering the functions of mitochondria. The version of p53 encoded by TP53 exon-6 truncating mutations lacked roughly half of the domains of the full-length p53 protein, specifically the domains that enable full-length p53 to enter the cell nucleus and bind DNA.
TP53 exon-6 truncating mutations occurred at higher than expected frequencies and produced proteins that lacked canonical p53 tumor suppressor activities but instead promoted cancer cell proliferation, survival, and metastasis. Functionally and molecularly, these p53 mutants resembled the naturally occurring alternative p53 splice variant, p53-psi. Due to their similarity to p53-psi, these mutants were able to localize to the mitochondria where they promoted tumor phenotypes by binding and activating the mitochondria inner pore permeability regulator protein, Cyclophilin D (CypD).
"Remarkably, despite 40 years of research and over 80,000 publications on p53, our new findings show that it still holds mystery and promise," said senior author Dr. Raffaella Sordella, an associate professor at Cold Springs Harbor Laboratory. "It seems that by changing mitochondrial function, the variants are priming cells to reprogram themselves. These mutations are strong candidates for targeting by precision medicine. The frequency of exon-6 truncating mutations in fact is comparable to other precision medicine targets such as the EGFR oncogenic-mutations found in lung cancer. We have begun discussing with several pharmaceutical companies ways in which we can use our newly gained knowledge to develop treatments that will make a positive difference for many cancer patients."
Related Links:
Cold Spring Harbor Laboratory














