Protein Identified Prevents Systemic Lupus Erythematosus
By LabMedica International staff writers
Posted on 11 Nov 2016
A protein has been identified that functions to prevent the immune system from generating the autoimmune response responsible for lupus erythematosus, a disease associated with inflammation of various organs including kidney, brain, skin, heart, and lung.Posted on 11 Nov 2016
Toll-like receptor 7 (TLR7) is a protein that plays an essential role in development of the autoimmune diseases systemic lupus erythematosus (SLE) by co-stimulating B-cells reactive to the endogenous TLR7 ligand Sm/ribonucleoprotein (RNP), a crucial lupus self-antigen. However, how the TLR7-mediated autoimmune response is regulated is not yet known.
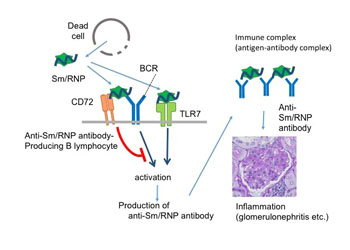
Image: The RNA-protein complex Sm/RNP, which is released from dead cells, stimulates B-cells capable of producing anti-Sm/RNP antibody (anti-Sm/RNP B lymphocytes). The resulting anti-Sm/RNP antibody induces development of systemic lupus erythematosus (SLE) by forming immune complexes together with Sm/RNP (Photo courtesy of the Department of Immunology, Tokyo Medical and Dental University).
To better understand the molecular mechanism that underlies SLE, investigators at Tokyo Medical and Dental University (Japan) genetically engineered mouse immune cells to modify or eliminate the inhibitory B-cell co-receptor protein CD72, which had been previously shown to prevent development of SLE.
They reported in the October 24, 2016, online edition of the Journal of Experimental Medicine that CD72 recognized Sm/RNP at the extracellular C-type lectin-like domain (CTLD) and specifically inhibited B-cell response to Sm/RNP. Moreover, the CTLD of CD72c, a lupus-susceptible allele, bound to Sm/RNP less strongly than that of lupus-resistant CD72a.
Reduced binding of CD72c was supported by x-ray crystallographic analysis that revealed a considerable alteration in charge at the putative ligand-binding site. Thus, CD72 appeared to specifically inhibit B-cell response to the endogenous TLR7 ligand Sm/RNP through CTLD-mediated recognition of Sm/RNP, thereby preventing production of anti-Sm/RNP antibody crucial for development of SLE.
"When we knocked out CD72 in mouse B-cells, they were specifically stimulated by the self-antigen Sm/RNP and released antibodies against this antigen," said senior author Dr. Takeshi Tsubata professor of immunology at Tokyo Medical and Dental University. "The lack of CD72 meant that another receptor on B-cells could bind to Sm/RNP, which activated the B-cells and led to the symptoms of SLE. We now know that CD72 prevents immune responses, which lead to SLE without affecting responses to microbes and cancer cells. If we can develop a method to augments capability of CD72, this will treat patients with SLE without unwanted effects."
Related Links:
Tokyo Medical and Dental University













