Laser-Heated Plasmonic Nanoparticles Safely Cause Localized Tumor Destruction
By LabMedica International staff writers
Posted on 16 Aug 2016
Cancer therapy based on plasmonic nanoparticles that homed to tumors, warmed on exposure to laser irradiation, and then selectively killed the cancer cells was optimized and shown to be effective in a mouse xenograft model.Posted on 16 Aug 2016
Plasmonic nanoparticle-based photothermal cancer therapy is a promising new tool to inflict localized and irreversible damage to tumor tissue by heating, without harming surrounding healthy tissue.
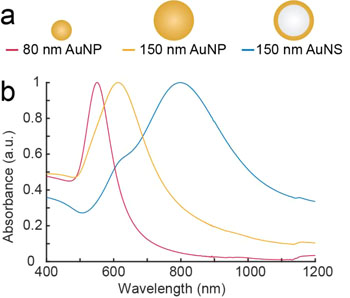
Image: The experiments were carried out with nanoparticles of different sizes and structures. The first two of the series consisted of solid gold and the last consisted of a core of glass with a surface of gold. The beads were illuminated with near infrared light with wavelengths of 807 nanometers and 1064 nanometers. The most effective nanoparticle was the gold-plated glass bead (Photo courtesy of Kamilla Nørregaard, University of Copenhagen).
Plasmonic resonance is a phenomenon that occurs when light is reflected off thin metal films, which may be used to measure interaction of biomolecules on the surface. An electron charge density wave arises at the surface of the film when light is reflected at the film under specific conditions. A fraction of the light energy incident at a defined angle can interact with the delocalized electrons in the metal film (plasmon) thus reducing the reflected light intensity. The angle of incidence at which this occurs is influenced by the refractive index close to the backside of the metal film, to which target molecules are immobilized. If ligands in a mobile phase running along a flow cell bind to the surface molecules, the local refractive index changes in proportion to the mass being immobilized. This can be monitored in real time by detecting changes in the intensity of the reflected light.
Investigators at the University of Copenhagen (Denmark) combined plasmonic resonance with nanoparticles made from gold or gold-covered glass as the basis for hyperthermic anticancer therapy. The effectiveness of the approach was assessed by PET (positron emission tomography).
The investigators described in the August 2, 2016, online edition of the journal Scientific Reports the quantification of heat generation and absorption cross-section of single irradiated nanoparticles using a temperature sensitive lipid-based assay that compared results to their theoretically predicted photo-absorption.
In vivo, the heat generation of irradiated nanoparticles was evaluated in human tumor xenografts in mice using 2-deoxy-2-[F-18]fluoro-D-glucose (18F-FDG) PET imaging. To validate the use of this platform, the investigators quantified the photothermal efficiency of near infrared resonant silica-gold nanoshells (AuNSs) and benchmarked this against the heating of colloidal spherical, solid gold nanoparticles (AuNPs). As expected, both in vitro and in vivo the heat generation of the resonant AuNSs performed better than the non-resonant AuNPs. Furthermore, the results showed that PET imaging could be reliably used to monitor early treatment response of photothermal treatment.
In the mouse study, 150 nanometer gold-covered glass nanoparticles were injected into tumors and then illuminated with near-infrared laser light, which penetrated through the tissue. In contrast to conventional radiation therapy, the near-infrared laser light caused no burn damage to the tissue that it passed through. Within an hour following the treatment, the investigators could detect cancer cell death on PET scans and determined that the effect continued for at least two days.
"The treatment involves injecting tiny nanoparticles directly into the cancer. Then you heat up the nanoparticles from outside using lasers. It is a strong interaction between the nanoparticles and the laser light, which causes the particles to heat up. What then happens is that the heated particles damage or kill the cancer cells," said senior author Dr. Lene Oddershede, professor of biophysics at the University of Copenhagen. "As physicists we have great expertise in the interaction between light and nanoparticles and we can very accurately measure the temperature of the heated nanoparticles. The effectiveness depends on the right combination between the structure and material of the particles, their physical size, and the wavelength of the light."
"Now we have proven that the method works. In the longer term, we would like the method to work by injecting the nanoparticles into the bloodstream, where they end up in the tumors that may have metastasized. With the PET scans we can see where the tumors are and irradiate them with lasers, while also effectively assessing how well the treatment has worked shortly after the irradiation. In addition, we will coat the particles with chemotherapy, which is released by the heat and which will also help kill the cancer cells," said Dr. Oddershede.
Related Links:
University of Copenhagen














