Blocking MicroRNA Synthesis Reverses Behavior of Tumor-Associated Macrophages
By LabMedica International staff writers
Posted on 22 Jun 2016
Preventing synthesis of microRNAs in compromised tumor associated macrophages (TAMs) reprograms these cells from being tumor supporting to being tumor suppressing.Posted on 22 Jun 2016
TAMs largely express an alternatively activated (or M2) phenotype, which entails immunosuppressive and tumor-promoting capabilities. Reprogramming TAMs towards a classically activated (M1) phenotype might reverse tumor-associated immunosuppression and activate anti-tumor immunity.
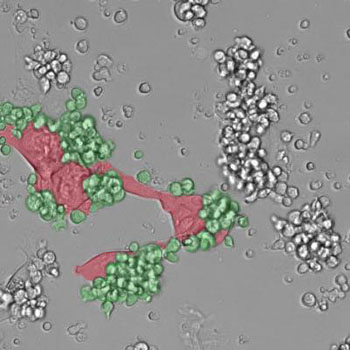
Image: A micrograph showing immune cells (green) attacking tumor cells (red) (Photo courtesy of Dr. Michele De Palma, Ecole Polytechnique Fédérale de Lausanne).
To test this possibility, investigators at Ecole Polytechnique Fédérale de Lausanne (Switzerland) used genetic engineering techniques to conditionally delete the microRNA (miRNA)-processing enzyme DICER1 in macrophages. The enzyme Dicer, which is encoded by the DICER1 gene, trims double stranded RNA, to form small interfering RNA (siRNA) or microRNA (miRNA). These processed RNAs are incorporated into the RNA-induced silencing complex (RISC), which targets messenger RNA to prevent translation.
The investigators reported in the June 13, 2016, online edition of the journal Nature Cell Biology that deletion of DICER1 prompted M1-like TAM programming, characterized by hyperactive IFN (interferon)-gamma/STAT1 (signal transducer and activator of transcription 1) signaling. This behavior modification eliminated the immunosuppressive capacity of TAMs and fostered the recruitment of activated cytotoxic T lymphocytes (CTLs) to the tumors. CTL-derived IFN-gamma increased the M1 polarization of DICER1-deficient TAMs and inhibited tumor growth.
Genetic rescue of Let-7 miRNA activity in DICER1-deficient TAMs partly restored their M2-like phenotype and decreased tumor-infiltrating CTLs. These findings suggested that DICER1/Let-7 microRNA activity opposed IFN-gamma-induced, immunostimulatory M1-like TAM activation.
"The most exciting finding was that TAM reprogramming greatly improved the efficacy of immunotherapy," said senior author Dr. Michele De Palma, a tenure track assistant professor at the Ecole Polytechnique Fédérale de Lausanne. "Our results in experimental models of cancer suggest a new therapeutic strategy based on inhibiting the microRNA machinery - or the Let-7 microRNAs - specifically in the TAMs, which may unleash the power of mainstream immunotherapies, such as immune checkpoint inhibitors."
Related Links:
Ecole Polytechnique Fédérale de Lausanne













