Supply of Lipid Precursors Modulates Growth of Breast Cancer Cells
By LabMedica International staff writers
Posted on 21 Apr 2016
An enzyme present in the cell membranes of breast cancer cells, which supplies lipid precursors that enable rapid cell growth, has emerged as a promising drug target.Posted on 21 Apr 2016
Investigators at the Institute for Research in Biomedicine (Barcelona, Spain) reported in the April 5, 2016, online edition of the journal Nature Communications that rapidly growing breast cancer cells were dependent on a mechanism to supply precursors for intracellular lipid production derived from extracellular sources, and they suggested that the enzyme endothelial lipase (LIPG) fulfilled this function.
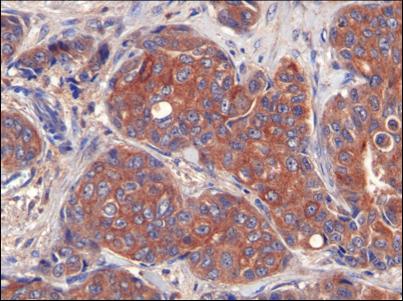
Image: A photomicrograph showing that breast tumor cells have high levels of LIPG (endothelial lipase) expression in their membranes (Photo courtesy of the Institute for Research in Biomedicine, Barcelona).
LIPG expression allowed the import of lipid precursors, thereby contributing to breast cancer proliferation. Analyses of more than 500 clinical samples from patients with various kinds of breast tumors revealed that 85% had high levels of LIPG expression. Thus, LIPG was identified as an essential component of the lipid metabolic adaptations that breast cancer cells, and not normal tissue, must undergo to support high proliferation rates.
The investigators found that LIPG expression was controlled by the signaling molecules FoxA1 (forkhead box protein A1) or FoxA2 (forkhead box protein A2) in all breast cancer subtypes. Experiments with breast cancer cell cultures and in animal models showed that downregulation of either LIPG or FoxA signaling resulted in decreased proliferation and impaired synthesis of intracellular lipids.
"This new knowledge related to metabolism could be the Achilles heel of breast cancer," said senior author Dr. Roger Gomis, oncology group leader at the Institute for Research in Biomedicine. "LIPG has many virtues as a target. If a drug were found to block its activity, it could be used to develop more efficient chemotherapy treatments that are less toxic than those currently available."
Related Links:
Institute for Research in Biomedicine













