Medical Implant Device Reduces Alzheimer’s Pathology and Provides Passive Immunization
By LabMedica International staff writers
Posted on 03 Apr 2016
In landmark proof-of-concept study with mice, a cutting-edge subcutaneous-implant capsule developed for treatment for Alzheimer’s disease, successfully directed the immune system against the disease.Posted on 03 Apr 2016
One of the hypothesized causes of Alzheimer’s is over-accumulation of the protein amyloid-beta (Abeta) in different areas of the brain, resulting in deposition of aggregated protein plaques, which are toxic to neurons. One of the most promising treatments is to tag Abeta with antibodies that signal the patient’s own immune system to attack and clear them. To be most effective, this treatment has to be given as early, before the first signs of cognitive decline. Another drawback is that this treatment requires repeated vaccine injections, which can cause side effects.
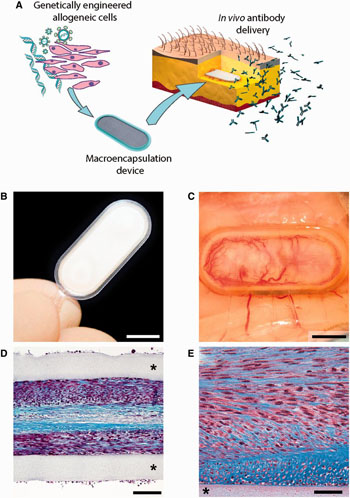
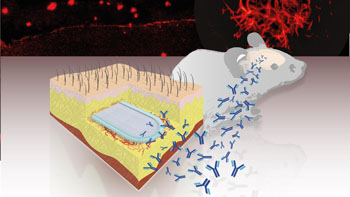
The laboratory of Patrick Aebischer at the Swiss Federal Institute of Technology in Lausanne (EPFL – École Polytechnique Fédérale de Lausanne; Switzerland) have now innovatively solved the problem. The team developed a bioactive capsule containing cells genetically engineered to produce antibodies against Abeta. The capsule is implanted in tissue under the skin and the cells release a steady and safe flow of anti-Abeta antibodies into the bloodstream, from where they cross over into the brain and target the Abeta plaques.
The “macroencapsulation device” is made of two permeable membranes sealed together with a polypropylene frame. The membranes also allow the cells to interact with surrounding tissue to get the nutrients needed. The complete device is 27-mm long, 12-mm wide, 1.2-mm thick, and contains a hydrogel that facilitates cell growth. All the materials used are biocompatible, and a method was specifically used that is easily reproducible for large-scale manufacturing.
The cells in the capsule must be able to produce antibodies, but they must not trigger a rejection response in immune system (as with organ transplants). This is where the capsule membranes come into play: they shield the cells from being identified and attacked by the immune system. This protection also enables cells from a single donor to be used on multiple patients.
In the study, the device was successful in reducing pathology when tested on a mouse model for Alzheimer’s. Treatment resulted in a dramatic reduction of Abeta plaque load, and the constant flow of antibodies produced by the capsule over a course of 39 weeks prevented the formation of Abeta plaques in the brain. The treatment also reduced phosphorylation of the protein tau, another sign of Alzheimer’s.
The work demonstrated a proof-of-concept that encapsulated cell implants can be used successfully and safely to deliver antibodies to treat Alzheimer’s disease and other neurodegenerative disorders that feature defective proteins.
The study, by Lathuilière A et al., was published March 8, 2016, in the journal Brain.
Related Links:
Swiss Federal Institute of Technology in Lausanne