Stem Cell Therapies Improve Survival of Mouse Brain Cancer Model
By LabMedica International staff writers
Posted on 09 Mar 2016
Two papers have described the use of fibrin-embedded, biologically engineered induced neural and mesenchymal stem cells in tumor-homing cytotoxic stem cell (SC) therapy that shows promise as a new approach for treating the incurable brain cancer glioblastoma multiforme (GBM).Posted on 09 Mar 2016
Investigators at the University of North Carolina (Chapel Hill, USA) used the process of transdifferentiation to convert skin fibroblasts into induced neural stem cells (iNSCs). Transdifferentiation, also known as lineage reprogramming, is a process where one mature somatic cell transforms into another mature somatic cell without undergoing an intermediate pluripotent state or progenitor cell type.
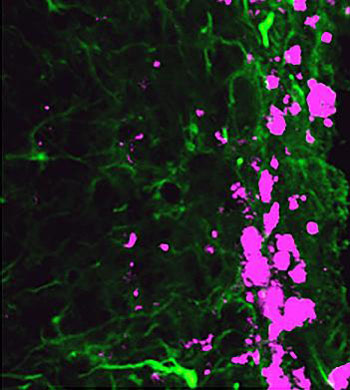
Image: Reprogrammed stem cells (green) chase down and kill glioblastoma cells (pink), potentially offering a new and more effective treatment option for a disease that has not had any in more than 30 years (Photo courtesy of the University of North Carolina).
They reported in the February 2, 2016, online edition of the journal Nature Communications that in a mouse brain cancer model, iNSCs were tumoritropic, homing rapidly to co-cultured glioblastoma cells and migrating extensively to distant tumor foci in the mouse brain. The iNSCs could be modified to deliver the anticancer molecule TRAIL (tumor necrosis factor-related apoptosis-inducing ligand). Treatment with iNSC-TRAIL decreased the growth of established solid and diffuse patient-derived glioblastoma xenografts 230- and 20-fold, respectively, while significantly prolonging the median mouse survival.
In the second paper, which was published in the January 6, 2016, online edition of the journal Biomaterials, the investigators described a fibrin-based transplant approach capable of increasing cytotoxic SC retention and persistence within the surgical cavity, yet remaining permissive to tumoritropic migration. Using the mouse GBM model, they showed that suspending human mesenchymal stem cells (hMSCS) in a fibrin matrix increased initial retention in the surgical resection cavity two-fold and prolonged persistence in the cavity three-fold compared to conventional delivery strategies.
Cytotoxic hMSCs in the fibrin matrix remained tumoritropic, rapidly migrating from the fibrin matrix to co-localize with cultured human GBM cells. When TRAIL (TR) was encapsulated into the hMSCs (hMSC-sTR) in a fibrin matrix, the hMSC-sTR/fibrin therapy reduced the viability of multiple three-dimensional human GBM spheroids and regressed established human GBM xenografts three-fold in 11 days.
In an experiment that mimicked the clinical therapy of surgically resected GBM, intra-cavity seeding of therapeutic hMSC-sTR encapsulated in fibrin reduced postsurgical GBM volumes six-fold, increased time to recurrence four-fold, and prolonged median survival from 15 to 36 days compared to control-treated animals.
"Our work represents the newest evolution of the stem-cell technology that won the Nobel Prize in 2012," said senior author Dr. Shawn Hingtgen, assistant of pharmacy at the University of North Carolina. "We wanted to find out if these induced neural stem cells would home in on cancer cells and whether they could be used to deliver a therapeutic agent. This is the first time this direct reprogramming technology has been used to treat cancer."
Related Links:
University of North Carolina













