Nanoparticle-Bound Paclitaxel Outperforms Abraxane in Mouse Cancer Models
By LabMedica International staff writers
Posted on 17 Aug 2015
A novel formulation that intercalated the toxic cancer drug paclitaxel within inert nanoparticles increased the potency of the drug in mouse models while reducing the severity of its adverse side effects.Posted on 17 Aug 2015
Investigators at Duke University (Durham, NC, USA) were looking for a form of the chemotherapeutic drug paclitaxel that would perform better than today's preferred formulation known as Abraxane. Abraxane, also called nab-paclitaxel, is a formulation where paclitaxel is bound to albumin nanoparticles. Much of the clinical toxicity of regular paclitaxel is associated with the solvent Cremophor EL in which it is dissolved for delivery. In Abraxane paclitaxel is bonded to albumin as an alternative delivery agent to the more toxic solvent delivery method. This formulation was approved by the [US] Food and Drug Administration (Bethesda, MD, USA) in January 2005 for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within six months of adjuvant chemotherapy
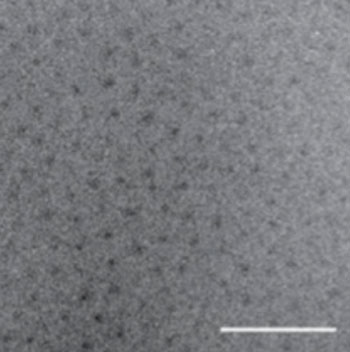
Image: Transmission electron micrograph (TEM) of the newly repackaged pharmaceutical. The dark spots are the water-insoluble cores of the nanoparticles, while the peptide chains are barely visible due to their low electron density and high degree of hydration (Photo courtesy of Dr. Ashutosh Chilkoti, Duke University).
The investigators worked with two mouse models: the first group of mice had human breast cancers growing in their own mammary glands, while the second group of mice had human prostate tumors growing under their skin. Both groups were treated with Abraxane or with a new formulation.
In this formation paclitaxel was conjugated to recombinant chimeric polypeptides (CPs) that spontaneously self-assembled into approximately 60 nanometer near-monodisperse nanoparticles that increased the systemic exposure of paclitaxel by sevenfold compared with the free drug and twofold compared with Abraxane. The tumor uptake of the nanoparticles was fivefold greater than the free drug and twofold greater than Abraxane.
Results published in the August 4, 2015, online edition of the journal Nature Communications revealed that in the mouse cancer models of human triple-negative breast cancer and prostate cancer, the paclitaxel nanoparticles induced near-complete tumor regression after a single dose in both tumor models, whereas at the same dose, no mice treated with Abraxane survived for more than 80 days (breast cancer) or 60 days (prostate cancer), respectively.
"The chemical bonds holding the polypeptide cage together are stable in blood, but dissolve in a tumor's lower pH levels," said first author Dr. Jayanta Bhattacharyya, a senior research scientist at Duke University. "This delivers the drug directly to the tumor and helps prevent it from randomly absorbing into healthy tissue, reducing side effects."
Related Links:
Duke University
[US] Food and Drug Administration














