Clinical Trials Demonstrate Potential of New Psoriasis Drug
By LabMedica International staff writers
Posted on 22 Jun 2015
Two clinical trials involving the candidate psoriasis drug ixekizumab showed that it was more effective than the currently used drug etanercept or a placebo.Posted on 22 Jun 2015
Ixekizumab is a humanized monoclonal antibody that selectively neutralizes interleukin 17A. This cytokine is now recognized as one of the primary factors behind the development of the characteristic red, scaly plaques of psoriasis. High levels of this cytokine have also been associated with several chronic inflammatory diseases including rheumatoid arthritis and multiple sclerosis.
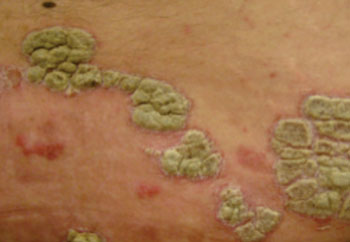
Image: Psoriasis plaques in the skin (Photo courtesy of Wikimedia Commons).
Investigators at the University of Manchester (United Kingdom) took part in two prospective, double-blind, multicenter, phase three studies (UNCOVER-2 and UNCOVER-3) that evaluated the efficacy of ixekizumab for treatment of psoriasis in comparison to etanercept or a placebo.
Etanercept is a fusion protein produced by recombinant DNA. It fuses the TNF (tumor necrosis factor) receptor to the constant end of the IgG1 antibody. It is a large molecule, with a molecular weight of 150,000 Daltons, which binds to TNFalpha and decreases its role in disorders involving excess inflammation in humans and other animals, including autoimmune diseases such as ankylosing spondylitis, juvenile rheumatoid arthritis, psoriasis, psoriatic arthritis, and rheumatoid arthritis. However, serious infections and sepsis, including fatalities, have been reported with the use of etanercept including reactivation of latent tuberculosis and hepatitis B infections.
Results published in the June 10, 2015, online edition of the journal the Lancet related to more than 2,500 psoriasis patients. Half were treated with ixekizumab once every two or four weeks, while the other half were given etanercept or a placebo.
Both ixekizumab dose regimens had greater efficacy than a placebo or etanercept over 12 weeks in the two independent studies. Around half of the patients treated with ixekizumab showed improvement as early as week four of the trial and up to 71% demonstrated a high level of improvement by week 12.
“The objective for treating psoriasis has been to reduce the visible symptoms,” said first author Dr. Christopher E M Griffiths, professor of dermatology at the University of Manchester. “But new drugs are fast showing us that a realistic goal for all patients should be attaining clear skin and this trial very much sets us on that path. What we saw in this trial was not just the physical aspects of the disease clearing up, but people on the new drug also reporting a marked improvement in their quality of life as they felt more confident and suffered less from itching – far more than in the other two groups.”
Related Links:
University of Manchester













