Preliminary Clinical Trial Demonstrates Feasibility of Treating Multiple Myeloma with the Patient's Own Marrow-Infiltrating Lymphocytes
By LabMedica International staff writers
Posted on 02 Jun 2015
Results of a small clinical trial support the feasibility of using a multiple myeloma patient's marrow-infiltrating lymphocytes (MILs) as the basis for adoptive T cell therapy (ACT).Posted on 02 Jun 2015
Investigators at Johns Hopkins University (Baltimore, MD, USA) hypothesized that MIL-based ACT in multiple myeloma could impart greater anti-tumor immunity in that they are obtained from the tumor microenvironment.
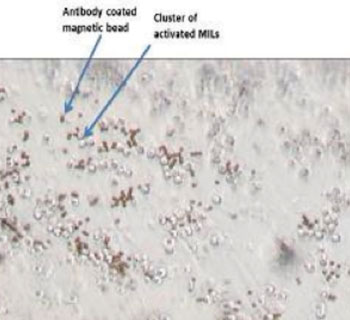
Image: Micrograph shows marrow-infiltrating lymphocytes in cell culture (Photo courtesy of Dr. Kimberly Noonan, Johns Hopkins University).
They discussed results from the first MILs ACT multiple myeloma clinical trial in the May 20, 2015, online edition of the journal Science Translational Medicine. For this study 22 patients with either newly diagnosed or relapsed disease had their MILs harvested, activated, and expanded with anti-CD3/CD28 beads plus interleukin-2, and subsequently re-infused on the third day following the standard regimen of high dose chemotherapy and stem cell transplant therapy.
Results revealed that seven patients experienced at least 90% reduction in tumor cell volume and survived, on average, 25.1 months without cancer progression. The remaining 15 patients had an average of 11.8 progression-free months following MILs therapy. Overall survival was 31.5 months for those with less than 90% disease reduction, while follow-up time is currently more than six years for those with a better response. None of the participants exhibited serious side effects from the MILs therapy.
"What we learned in this small trial is that large numbers of activated MILs can selectively target and kill myeloma cells," said senior author Dr. Ivan Borrello, professor of oncology at Johns Hopkins University. "Several US cancer centers have conducted similar experimental treatments, known as adoptive T cell therapy, but the Johns Hopkins team is apparently the only one to use MILs. Other types of tumor-infiltrating cells can be used, but they are usually less plentiful in patients' tumors and may not grow as well outside the body."
Related Links:
Johns Hopkins University













