Two Secreted Proteins Prepare Breast Cancer Cells for Metastasis
By LabMedica International staff writers
Posted on 22 Apr 2015
Two genes that code for secreted proteins have been found to be responsible for the development of vascular mimicry—imitation blood vessels—by tumor cells and for preparing some tumor cell clones to spread away from the site of the primary tumor.Posted on 22 Apr 2015
Investigators at Cold Spring Harbor Laboratory (NY, USA) and colleagues at the University of Cambridge (United Kingdom) studied the two genes, SERPINE2 (serpin peptidase inhibitor, clade E) and SLPI (secretory leukocyte peptidase inhibitor), in a polyclonal mouse breast tumor model.
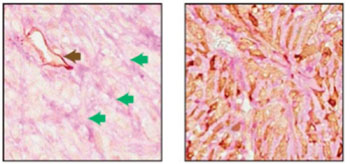
Image: Two adjacent sections of a mouse breast tumor. The tissue at left was stained so that normal blood vessels can be seen (black arrow); extending from these vessels are blood filled channels (green arrows). On the right, the tissue was stained for a fluorescent protein expressed by the tumor cells. Here it is seen that blood-filled channels were actually formed by tumor cells in a process known as vascular mimicry (Photo courtesy of Cold Spring Harbor Laboratory).
The SERPINE 2 gene encodes a member of the serpin family of proteins, a group of proteins that inhibit serine proteases. Thrombin, urokinase, plasmin, and trypsin are among the proteases that this family member can inhibit. This gene is a susceptibility gene for chronic obstructive pulmonary disease and for emphysema. The SLPI gene encodes a secreted inhibitor which protects epithelial tissues from serine proteases. It is found in various secretions including seminal plasma, cervical mucus, and bronchial secretions, and has affinity for trypsin, leukocyte elastase, and cathepsin G. Its inhibitory effect contributes to the immune response by protecting epithelial surfaces from attack by endogenous proteolytic enzymes; the protein is also thought to have broad-spectrum antibiotic activity.
The investigators found that breast tumors in their mouse model comprised distinct clones whose component cells specialized in various functions such as dominating the primary tumor, contributing to metastatic populations, or showing tropism for entering the lymphatic or vasculature systems. The investigators were able to correlate these stable properties with distinct gene expression profiles. Those clones that efficiently entered the vasculature expressed the two secreted proteins, Serpine2 and Slpi, which were necessary and sufficient to program these cells for vascular mimicry. The data, which was published in the April 8, 2015, online edition of the journal Nature, indicated that these proteins not only drove the formation of extravascular networks but also ensured their perfusion by acting as anticoagulants.
The investigators proposed that vascular mimicry supported the ability of some breast tumor cells to contribute to distant metastases while simultaneously satisfying a critical need of the primary tumor to be fed by the vasculature. Enforced expression of SERPINE2 and SLPI in human breast cancer cell lines also programmed them for vascular mimicry, and SERPINE2 and SLPI were overexpressed preferentially in human patients that had lung-metastatic relapse. Thus, these two secreted proteins, and the phenotype they promote, may be broadly relevant as drivers of metastatic progression in human cancer.
"It is very neat to watch and see cells evolve to have these capacities," said senior author Dr. Simon Knott, research professor at Cold Spring Harbor Laboratory. "But on the other hand it is really scary to think that these cells are sitting there in people doing this. Targeting them might provide therapeutic benefits, but we are not sure yet."
Related Links:
Cold Spring Harbor Laboratory
University of Cambridge









 Analyzer.jpg)



