Methodology Devised to Improve Stem Cell Reprogramming
By LabMedica International staff writers
Posted on 28 Jan 2015
In a study that provides scientists with a critical new determination of stem cell development and its role in disease, researchers have established a first-of-its-kind approach that outlines the stages by which specialized cells are reprogrammed into stem cells resembling those found in embryos. The research could have wide ranging, long-term implications in enhancing disease modeling and devising new therapies for patients.Posted on 28 Jan 2015
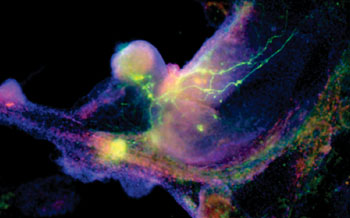
The study, conducted by researchers from the University of California, Los Angeles (UCLA; USA) Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research and led by Dr. Kathrin Plath, a professor of biological chemistry, was published January 2015 in the journal Cell. Induced pluripotent stem cells (iPSCs) are cells that can be generated from adult cells and then, like embryonic stem cells, be directed to become any cell in the human body. Adult cells can also be reprogrammed in the lab to change from a specialized cell back to an iPSC (and thereby becoming a cell similar to that of an embryonic stem cell).
Reprogramming takes one to two weeks and is a mostly inefficient process, with typically less than one percent of the beginning cells effectively becoming an iPSC. The exact stages a cell goes through during the reprogramming process are not well understood. This knowledge is vital, because iPSCs have great potential in the field of regenerative medicine, as they can constantly reproduce and provide a single source of patient-specific cells to replace those lost to injury or disease. They can also be used to create innovative disease models from which new drugs and therapies can be developed.
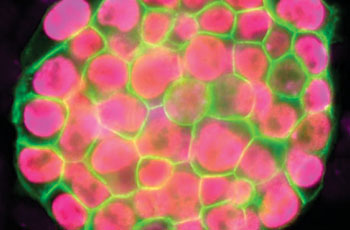
Vincent Pasque and Jason Tchieu, postdoctoral fellows in Plath’s lab and co-first authors of the study, developed a roadmap of the reprogramming process using detailed time-course analyses. They induced the reprogramming of specialized cells (that could only make more of themselves, and no other cell types), then observed and analyzed on a daily basis or every other day the process of transformation at the single-cell level. The data were gathered and recorded during a time period of up to two weeks.
Dr. Plath’s team found that the changes that happen in cells during reprogramming occur in sequentially, and that notably, the stages of the sequence were the same across the diverse reprogramming systems and different cell types analyzed. “The exact stage of reprogramming of any cell can now be determined,” Dr. Pasque said. “This study signals a big change in thinking, because it provides simple and efficient tools for scientists to study stem cell creation in a stage-by-stage manner. Most studies to date ignore the stages of reprogramming, but we can now seek to better understand the entire process on both a macro and micro level.”
Dr. Plath’s group additionally discovered that the stages of reprogramming to iPSC are different from what was expected. They found that it is not simply the reversed sequence of stages of embryo development. Some steps are reversed in the expected order; others do not actually happen in the exact reverse order and resist a change until late during reprogramming to iPSCs. “This reflects how cells do not like to change from one specialized cell type to another and resist a change in cell identity,” Dr. Pasque said. “Resistance to reprogramming also helps to explain why reprogramming takes place only in a very small proportion of the starting cells.”
With these findings, Dr. Plath’s lab plans future studies to actively isolate specific cell types during specific stages of reprogramming. They also hope the research will encourage further investigation into the characteristics of iPSC development. “This research has broad impact, because by understanding cell reprogramming better we have the potential to improve disease modeling and the generation of better sources of patient-specific specialized cells suitable for replacement therapy,” concluded Dr. Plath. “This can ultimately benefit patients with new and better treatments for a wide range of diseases.”
Related Links:
University of California, Los Angeles’ Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research










 (3) (1).png)


