Acoustic Device Designed to Separate Tumor Cells from Blood Cells Could Help Assess Cancer’s Spread
By LabMedica International staff writers
Posted on 24 Sep 2014
Researchers have devised a new way to separate cells by exposing them to sound waves as they flow through a tiny channel. Their device, approximately the size of a dime, could be used to detect the extremely rare tumor cells that circulate in cancer patients’ blood, helping clinicians predict whether a tumor is going to metastasize.Posted on 24 Sep 2014
Separating cells with sound offers a milder option to existing cell-sorting technologies, which require tagging the cells with chemicals or exposing them to stronger mechanical forces that may damage them. “Acoustic pressure is very mild and much smaller in terms of forces and disturbance to the cell. This is a most gentle way to separate cells, and there’s no artificial labeling necessary,” said Dr. Ming Dao, a lead research scientist in Massachusetts Institute of Technology’s (MIT; Cambridge, MA, USA) department of materials and engineering, and one of the senior authors of the paper, which was published in September 2014 in the journal Proceedings of the National Academy of Sciences of the United States of America (PNAS).
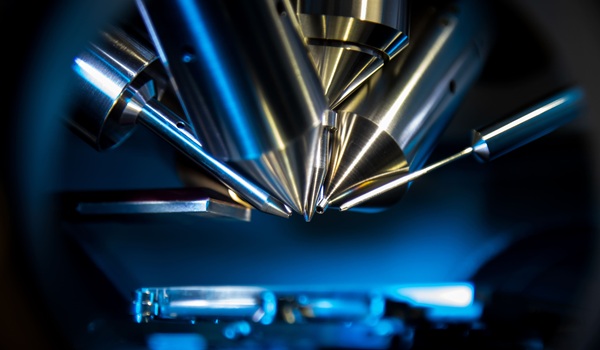
![Image: Microfluidic device uses sound waves to sort tumor from white-blood cells as they flow through the channel from left to right (IDT [interdigital transducers] are sound source) (Photo courtesy of Ding X, et al). Image: Microfluidic device uses sound waves to sort tumor from white-blood cells as they flow through the channel from left to right (IDT [interdigital transducers] are sound source) (Photo courtesy of Ding X, et al).](https://globetechcdn.com/mobile_labmedica/images/stories/articles/article_images/2014-09-23/JQR-695.jpg)
Image: Microfluidic device uses sound waves to sort tumor from white-blood cells as they flow through the channel from left to right (IDT [interdigital transducers] are sound source) (Photo courtesy of Ding X, et al).
Subra Suresh, president of Carnegie Mellon University (Pittsburgh, PA, USA), a professor of engineering emeritus, and a former dean of engineering at MIT, and Tony Jun Huang, a professor of engineering science and mechanics at Pennsylvania State University (Penn State; University Park, USA), are also senior authors of the article. The researchers have filed for a patent on the device; the technology of which they have demonstrated can be used to separate rare circulating cancer cells from white blood cells.
To sort cells using sound waves, scientists have previously built microfluidic devices with two acoustic transducers, which produce sound waves on either side of a microchannel. When the two waves meet, they combine to form a standing wave (a wave that remains in constant position). This wave generates a pressure node (line of low pressure) running parallel to the direction of cell flow. Cells that encounter this node are moved to the side of the channel; the distance of cell movement depends on their size and other properties such as compressibility.
However, these existing devices are inefficient: Because there is only one pressure node, cells can be pushed aside only short distances. The new device overcomes that obstacle by tilting the sound waves so they run across the microchannel at an angle: meaning that each cell encounters several pressure nodes as it flows through the channel. Each time it encounters a node, the pressure guides the cell a little further off center, making it simpler to capture cells of different sizes by the time they reach the end of the channel.
This simple modification drastically increases the efficiency of such devices, according to Taher Saif, a professor of mechanical science and engineering at the University of Illinois at Urbana-Champaign (USA). “That is just enough to make cells of different sizes and properties separate from each other without causing any damage or harm to them,” said Prof. Saif, who was not involved in this work.
In this study, the researchers first assessed the system with plastic beads, finding that it could separate beads with diameters of 9.9 and 7.3 micrometers with approximately 97% accuracy. They also devised a computer simulation that can predict a cell’s trajectory through the channel based on its size, density, and compressibility, as well as the angle of the sound waves, allowing them to tailor the device to separate different types of cells.
To evaluate whether the device could be useful for detecting circulating tumor cells, the researchers tried to separate breast cancer cells known as MCF-7 cells from white blood cells. These two cell types differ in size (20 micrometers in diameter for MCF-7 and 12 micrometers for white blood cells), as well as density and compressibility. The device successfully recovered about 71% of the cancer cells; the researchers plan to test it with blood samples from cancer patients to see how well it can detect circulating tumor cells in clinical settings. Such cells are very scarce; a 1-mL sample of blood may contain only a few tumor cells.
“If you can detect these rare circulating tumor cells, it’s a good way to study cancer biology and diagnose whether the primary cancer has moved to a new site to generate metastatic tumors,” Dr. Dao stated.
“This method is a step forward for detection of circulating tumor cells in the body. It has the potential to offer a safe and effective new tool for cancer researchers, clinicians, and patients,” Dr. Suresh concluded.
Related Links:
Massachusetts Institute of Technology
Carnegie Mellon University
Pennsylvania State University









 (3) (1).png)