Hair Restoration Method Clones Patients’ Cells to Grow New Hair Follicles
By LabMedica International staff writers
Posted on 09 Sep 2014
Researchers have developed of a new hair restoration approach that uses a patient’s cells to grow new hair follicles. In addition, the [US] Food and Drugs Administration (FDA) recently approved a new drug found to restore hair in patients with Alopecia areata.Posted on 09 Sep 2014
Researchers have identified the immune cells responsible for destroying hair follicles in people with alopecia areata, a common autoimmune disease that causes hair loss, and have evaluated an FDA-approved drug that eliminated these immune cells and restored hair growth in a small number of patients.
The study’s findings were published in August 17, 2014, online issue of the journal Nature Medicine. In the article, the researchers reported early findings from an ongoing clinical trial of the drug, which has produced complete hair regrowth in several patients with moderate-to-severe alopecia areata. Data from three participants were presented in the current paper; each patient experienced total hair regrowth within five months of the start of treatment.
“We’ve only begun testing the drug in patients, but if the drug continues to be successful and safe, it will have a dramatic positive impact on the lives of people with this disease,” said Raphael Clynes, MD, PhD, who led the research, along with Angela M. Christiano, PhD, professor in the departments of dermatology and of genetics and development at Columbia University (New York, NY, USA).
Alopecia areata is a common autoimmune disease that causes disfiguring hair loss. The disease can occur at any age and affects men and women equally. Hair is often lost in patches on the scalp, but in some patients it also causes loss of facial and body hair. There are no known treatments that can completely restore hair, and patients with the disease experience significant psychological stress and emotional suffering.
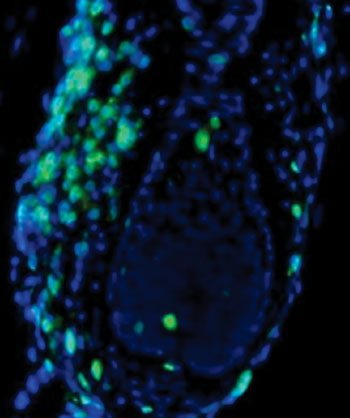
Scientists have known for a long time that hair loss in alopecia areata occurs when cells from the immune system surround and attack the base of the hair follicle, causing the hair to fall out and enter a dormant state. Until now, the specific type of cell responsible for the attack had been a mystery. Key evidence was discovered four years ago in Prof. Christiano’s genetic study of more than 1,000 patients with the disease. That study suggested that a “danger signal” in the hair follicles of patients—not previously linked to alopecia areata— entices the immune cells to the follicle and sparks the attack.
In the study, the investigators first studied mice with the disease, and then tracked backward from the danger signal to identify the specific set of T cells responsible for attacking the hair follicles. Further investigation of mouse and patient cells revealed how the T cells are instructed to attack and identified several key immune pathways that could be targeted by a new class of drugs, known as JAK inhibitors.
Two FDA-approved JAK inhibitors tested separately by the researchers—ruxolitinib and tofacitinib—were able to block these immune pathways and stop the attack on the hair follicles. In mice with extensive hair loss from the disease, both drugs completely restored the animals’ hair within 12 weeks. Each drug’s effect was also long-lasting, as the new hair persisted for several months after stopping treatment.
Together with Julian Mackay-Wiggan, MD, MS, director of the clinical research unit in the department of dermatology at CUMC and a practicing dermatologist at NewYork-Presbyterian/Columbia who treats patients with multiple types of hair loss, the researchers rapidly initiated a small open-label clinical trial of ruxolitinib, which is FDA approved for the treatment of a blood disorder, in patients with moderate-to-severe alopecia areata (with more than 30% hair loss). In three of the trial’s early participants, ruxolitinib totally restored hair growth within four to five months of starting treatment, and the attacking T cells disappeared from the scalp.
“We still need to do more testing to establish that ruxolitinib should be used in alopecia areata, but this is exciting news for patients and their physicians,” Dr. Clynes said. “This disease has been completely understudied, until now, only two small clinical trials evaluating targeted therapies in alopecia areata have been performed, largely because of the lack of mechanistic insight into it.”
“The timeline of moving from genetic findings to positive results in a clinical trial in only four years is astoundingly fast and speaks to this team’s ability to perform translational science of the highest caliber,” said David Bickers, MD, a professor of dermatology and chair of the department of dermatology at CUMC and dermatologist-in-chief at NewYork-Presbyterian/Columbia (New York, NY, USA). A practicing dermatologist who cares for many patients with alopecia areata himself, Dr. Bickers said, “There are few tools in the arsenal for the treatment of alopecia areata that have any demonstrated efficacy. This is a major step forward in improving the standard of care for patients suffering from this devastating disease.”
Columbia University has filed for intellectual property protection on the treatment of alopecia areata with small-molecule JAK inhibitors.
In similar hair loss research, Prof. Christiano and her Columbia University colleagues reported that other restoration techniques would simply redistribute from one area of the scalp to another, but a new application expands the use of hair transplants for female hair loss sufferers and men in the early stages of male pattern baldness.
The technique both creates more follicles and rejuvenates existing ones, beginning with cells grown from just a few hundred donor hairs, making hair transplants available to those with very few follicles, including patients with female-pattern hair loss, hair loss caused burns and scarring alopecia.
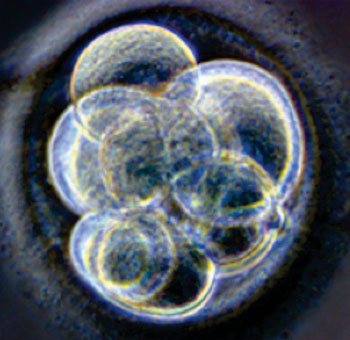
Colin Jahoda, from Durham University (UK) commented that dermal papilla cells, found in human skin, can form hair follicles. The idea of using these cells to clone hair follicles has been around since the 1970s.
Prof. Christiano and the other researchers harvested dermal papillae from several human donors and cloned cells in tissue culture, regulating additional growth factors, then transplanted the cultured papillae between the skin’s dermis and epidermis, grafted onto the backs of mice. These papillae transplants caused new hair growth that lasted at least six weeks in five of the seven tests.
Related Links:
Columbia University