Barth Syndrome Stem Cells Reveal Details of a Rare Heart Defect
By LabMedica International staff writers
Posted on 28 May 2014
Skin cells taken from Barth syndrome patients were used to generate stem cells that differentiated into defective heart tissue in culture.Posted on 28 May 2014
Barth syndrome (type II 3-Methylglutaconic aciduria) is caused by mutation of the tafazzin gene. Tafazzin is responsible for remodeling of a phospholipid cardiolipin (CL), the signature lipid of the mitochondrial inner membrane. As a result, Barth syndrome patients exhibit defects in CL metabolism, including aberrant CL fatty acyl composition, accumulation of monolysocardiolipin (MLCL), and reduced total CL levels. About 120 cases of Barth syndrome, which is found exclusively in males, have been documented to date, but the syndrome is believed to be severely under-diagnosed and has been estimated to occur in one out of approximately 300,000 births.
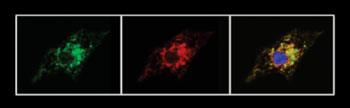
Image: The series of images shows how inserting modified RNA into diseased cells causes the cells to produce functioning versions of the TAZ protein (from left, green), which correctly localize in the mitochondria (red). When the images are merged to demonstrate this localization, green overlaps with red, giving the third image a yellow color around its edges (Photo courtesy of Harvard University).
Investigators at Harvard University (Cambridge, MA, USA) obtained skin cells from two Barth syndrome patients. The skin cells were induced to become stem cells carrying the patients’ TAZ mutations. The stem cells were cultured on chips lined with human extracellular matrix (ECM) proteins that mimicked their natural environment. Under these conditions the stem cells matured into a conglomerate of cardiomyocytes that mimicked heart tissue. Due to the presence of the TAZ mutations the heart tissue demonstrated very weak contractions, similar to a diseased human heart.
The investigators used this novel model system to define metabolic, structural, and functional abnormalities associated with TAZ mutation. They found that excess levels of reactive oxygen species (ROS) mechanistically linked TAZ mutation to impaired cardiomyocyte function. In addition, they used a gene therapy technique to provide the normal TAZ protein to the diseased tissue. Results published in the May 11, 2014, online edition of the journal Nature Medicine showed that inducing TAZ mutation in normal cardiomyocytes weakened contractions while addition of normal TAZ to the Barth syndrome cardiomyocytes corrected the contractile defect.
“The TAZ mutation makes Barth syndrome cells produce an excess amount of reactive oxygen species, or ROS—a normal byproduct of cellular metabolism released by mitochondria—which had not been recognized as an important part of this disease,” said senior author Dr. William Pu, associate professor of cardiology at Harvard University. “We showed that, at least in the laboratory, if you quench the excessive ROS production then you can restore contractile function. “Now, whether that can be achieved in an animal model or a patient is a different story, but if that could be done, it would suggest a new therapeutic angle.”
Related Links:
Harvard University














