Brain Buffer System Overcomes Molecular Disturbances in Circadian Clock
By LabMedica International staff writers
Posted on 01 May 2014
New evidence has been found for neuronal network communication that helps create a behavioral buffer in the brain to overcome certain disturbances in the molecular level circadian clock rhythms.Posted on 01 May 2014
Circadian clocks time the sleep/wake cycles as well as many other physiological and cellular pathways to daily 24 hour rhythms. In Drosophila, CLOCK (CLK) and CYCLE (CYC) proteins initiate the circadian system by promoting rhythmic transcription of hundreds of genes. Abolishment of circadian transcriptional oscillations (CTOs) has been shown to abolish circadian function. However, previous studies used manipulations in which the abolishment of the CTOs was very dramatic and involved strong up- or down-regulation of circadian genes.
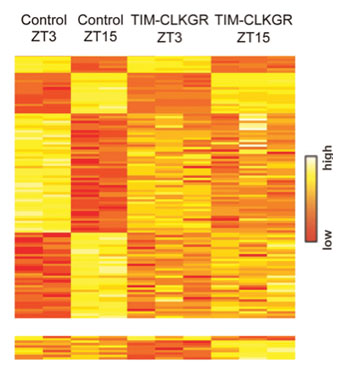
Image: Heat plots for microarray transcriptome experiment, of the genetically engineered Drosophila flies, indicating that the reduced amplitudes of the CLK-protein-driven circadian transcription oscillations (CTOs) leads to genome-wide reductions in CTO amplitudes, not only of the direct CLK-driven transcripts (Photo courtesy of Prof. Kadener, Hebrew University - Jerusalem, Israel, and PLOS Genetics).
In this study, a research team led by Sebastian Kadener, assistant professor at the Hebrew University of Jerusalem (Israel), used an innovative genetic approach that enabled them to generate Drosophila melanogaster fruit flies in which the amplitude of CLK-driven CTOs was reduced in a controlled way, either partially (approx. 60%) or strongly (90%). To the best of their knowledge, this is the first time CTOs have been partially damped in a living organism and their role assessed comprehensively.
The researchers postulated that in the brain, communication among the circadian neuronal groups can compensate for the dampened CTOs. This is not surprising, as results from studies on locomotor activity patterns in mammals with core clock protein mutations are among the same lines. However, in mammals the molecular machinery that drives circadian rhythms in the central versus the peripheral oscillators differs, whereas this does not seem to be the case in flies. Yet, in this study, the partial decrease in the amplitude of CTOs led to impaired function of circadian outputs in peripheral functions but did not significantly affect circadian locomotor behavior. This suggests that the clock in the brain has a specific compensatory mechanism. Moreover, flies with reduced CTOs that also had impaired circadian neuronal communication displayed aberrant circadian behavior rhythms.
The partially reduced CTOs led to low amplitude circadian protein oscillations (CPOs) that were not sufficient to drive outputs of peripheral oscillators, while circadian rhythms in locomotor activity were resistant to these partial reductions. This resilience of the brain oscillator was found to depend on communication among circadian neurons in the brain. Indeed, the capacity of the brain oscillator to overcome the low amplitude CTOs depends on the action of the neuropeptide PDF and on the pdf-expressing cells having equal or higher amplitude of CTOs than the rest of the circadian neuronal groups in the brain.
These findings support the idea of network buffering mechanisms that allows the brain to drive robust behavioral circadian rhythms even with low amplitude molecular oscillations. Therefore, in addition to revealing the importance of high amplitude CTOs for cell-autonomous circadian timekeeping, this work demonstrates that the brain's circadian neuronal network has an essential system that protects against disturbances in circadian transcription in the brain.
The study, by Weiss R. et al., was described in the journal PLOS Genetics, published April 3, 2014.
Related Links:
Hebrew University of Jerusalem













