Cytokines Induced by Exposure to Airborne Pollutants Drive the Transition from Acute to Chronic Lung Infections
By LabMedica International staff writers
Posted on 28 Aug 2013
Molecular signaling molecules that link acute viral infections with the development of chronic diseases such as chronic obstructive pulmonary disease (COPD) have been identified in a mouse model and in human patient samples.Posted on 28 Aug 2013
COPD is considered to be the fifth leading cause of death worldwide. It is characterized by inflammation of the lower airways and destruction of lung tissue that limit airflow and pulmonary function. While exposure to cigarette smoke is a major risk factor for COPD, response to viral infection by cells lining the airways can lead to the long-term lung inflammation and mucus production that are typical of COPD.
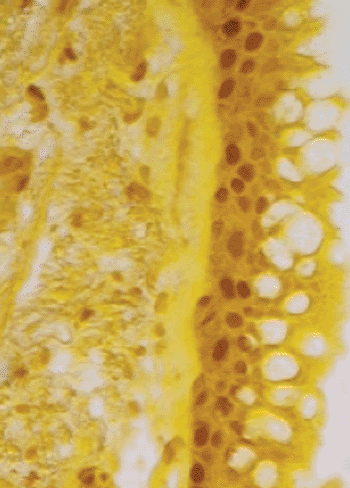
Image: This photomicrograph depicts airway epithelial cells from lung tissue of a COPD patient. The cell nuclei have been stained to reveal IL-33, a type of signaling molecule found at high levels in COPD patients. New research shows that viral infection can induce these cells to proliferate. Release of IL-33 from these cells promotes inflammatory mucus production. These findings provide insight into the mechanisms linking acute infection to chronic inflammatory lung disease (Photo courtesy of Holtzman Lab, Washington University School of Medicine).
Investigators at Washington University School of Medicine (St. Louis, MO, USA) had shown previously in mice with parainfluenza virus infection that innate immune cells played an unexpected role in interleukin-13 (IL-13)–dependent chronic lung disease. However, it was not known how IL-13 activity was modulated.
In the current report, the investigators demonstrated that lung levels of IL-33 were selectively increased in postviral mice with chronic obstructive lung disease and in humans with very severe COPD. IL-33 is a cytokine belonging to the IL-1 superfamily that induces helper T-cells, mast cells, eosinophils, and basophils to produce type II cytokines. IL-33 mediates its biological effects by interacting with the receptors ST2 (IL1RL1) and IL-1 Receptor Accessory Protein (IL1RAP), activating intracellular molecules in the NF-kappaB and MAP kinase signaling pathways that drive production of type II cytokines (e.g. IL-5 and IL-13) from polarized Th2 cells. The induction of type II cytokines by IL-33 in vivo is believed to induce the severe pathological changes observed in mucosal organs following administration of IL-33.
In humans with COPD, IL-33 gene expression was also associated with IL-13 and mucin gene expression, and IL-33 induction was traceable to a subset of airway basal cells with increased capacities for pluripotency and ATP-regulated release of IL-33.
“From this work, we now know that a respiratory viral infection leads to an increase in lung epithelial progenitor cells that are programmed for increased production of IL-33,” said senior author Dr. Michael J. Holtzman, professor of medicine at Washington University School of Medicine. “We also provided the initial evidence that an additional stress or danger, such as smoking or pollution or even another infection, could cause these cells to release IL-33, which then stimulates immune cells to produce IL-13 and in turn the airway mucus typical of COPD and related respiratory diseases. It is also possible that smoke exposure predisposes individuals to the development of these cells and, in turn, the susceptibility to exacerbation and progression of this type of disease.”
“The innate immune response is conventionally viewed as built for short- rather than long-term activation,” said Dr. Holtzman. “So the type of pathway that we identified was thought to be activated for only short periods of time. However, we found that it could be persistently activated after viral infection and became even more active with time.”
The study was published in the August 15, 2013, online edition of the Journal of Clinical Investigation.
Related Links:
Washington University School of Medicine













