Loss of Critical Enzyme Prevents Infection by Mutant Leishmania Parasite
By LabMedica International staff writers
Posted on 27 Jul 2010
A group of enzymes that are key regulators of cell growth, proliferation, and structure in eukaryotes has been cited as potential targets by drug developers seeking a cure for the parasitic disease leishmaniasis.Posted on 27 Jul 2010
Leishmaniasis is a parasitic disease with cutaneous, mucocutaneous, and visceral clinical manifestations, depending on the Leishmania spp. and human host. Worldwide, there are some 350 million people at risk of contracting the disease, but current treatment options rely predominantly on outmoded pentavalent antimonials, which have the potential to cause serious systemic toxicity.
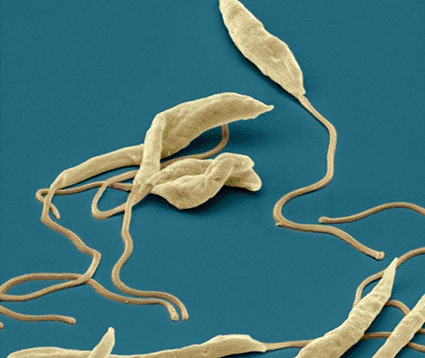
Image: Colored scanning electron micrograph (SEM) of several Leishmania parasitic protozoans (Photo courtesy of the Eye of Science).
Investigators at Washington University School of Medicine (St. Louis, MO, USA) searched genome databases and located the genes that encode three TOR (target of rapamycin) kinases in Leishmania major. In all eukaryotic organisms, these enzymes are linked to the regulation of critical cell events such as growth, proliferation, and structural maintenance.
The investigators attempted to engineer genetically L. major variants lacking the gene for one or more of the three TOR enzymes. They reported in the June 20, 2010, online edition of the journal Proceedings of the [U.S.] National Academy of Sciences (PNAS) that the parasite could not survive removal of the genes for either TOR kinase-1 or TOR kinase-2. On the other hand, removal of TOR kinase-3 (while leaving the other two intact) resulted in a slower growing mutant that maintained normal morphology, rapamycin sensitivity, and differentiation into the animal-infective promastigote stage. Significantly, these mutants were unable to survive or replicate in macrophages in vitro, or to induce pathology or establish infections in mice in vivo. The loss of virulence was associated with a defect in acidocalcisome formation, as this unique organelle was grossly altered in the TOR kinase -3 mutants and it no longer accumulated polyphosphates. The mutants also showed defects in osmoregulation and were sensitive to starvation for glucose but not amino acids.
Results of this study indicate that acidocalcisomes are essential for infection and may modulate the flow of fluids across the cell membrane or provide a mechanism for coping with stress and glucose depletion.
"If we can hit any of these proteins with a drug that will inhibit them, we should be able to strike a significant blow against Leishmania,” said senior author Dr. Stephen Beverley, professor of molecular microbiology at Washington University School of Medicine. "Given the numerous inhibitors already available, I think there is a pretty good chance that we will be able to identify a compound that specifically inhibits one of Leishmania's TOR kinases.”
Related Links:
Washington University School of Medicine














