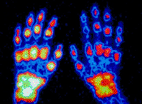
Gene Variants Predict Susceptibility to and Severity of Rheumatoid Arthritis
By Biotechdaily staff writers
Posted on 27 Sep 2007
Posted on 27 Sep 2007
Image
Carriers of the risk variants (about 65-70% of the general population) had an approximately 37% increased risk for developing RA compared with non-carriers. The novel variants appear in the TRAF1/C5 gene region.
Scientists from Celera (Rockville, MD, USA), an Applera Corp. business, and its collaborators at the Leiden University Medical Centre (The Netherlands), and the Karolinska Institute (Stockholm, Sweden) evaluated DNA samples from more than 4,700 individuals to compare patterns of genetic variation in those with rheumatoid arthritis to those without the disease. TRAF1/C5 was confirmed in four independent research studies (two in the Netherlands and one each in Sweden and the United States), and was responsible for about 6% of the risk for this disease. Located on chromosome 9, TRAF1 encodes a protein that is a member of the tumor necrosis factor (TNF) receptor-associated factor (TRAF) protein family, which associates with, and mediates the signal transduction from, various receptors of the TNF superfamily. C5 encodes a key component of the complement system and activation of the complement system has been implicated in the pathogenesis of many inflammatory diseases.
The findings from the four independent studies involving more than 4,700 individuals are likely to improve identification of high-risk individuals enabling earlier therapeutic intervention.
Celera first confirmed that this region was associated with RA with its discovery that PTPN22 is a genetic contributor to the etiology of RA. Another major contributor to RA risk is human leukocyte antigen (HLA). The discovery of TRAF1/C5 provides a valuable addition to HLA and PTPN22, which contribute approximately 30% and 12%, respectively, in explaining a genetic contribution to RA.
"Celera is currently determining the clinical utility of these gene variants and integrating them with other genetic markers for early diagnosis, prediction of disease severity, and possibly a patient's response to various therapies in rheumatoid arthritis,” said Thomas White, Ph.D., chief scientific officer at Celera. "Our goal at Celera is to incorporate these discoveries into new diagnostic tests, and initial data suggest an association with increased disease progression. From a functional perspective, it is interesting that TRAF1 is likely involved in TNF signaling, and we are looking to identify the role it may play in predicting the efficacy of drugs that work via this pathway.”
Related Links:
Celera
Leiden University Medical Centre
Karolinska Institute













