Rapid Test for Chlamydia Antigen Antibodies Optimized
|
By LabMedica International staff writers Posted on 07 May 2019 |
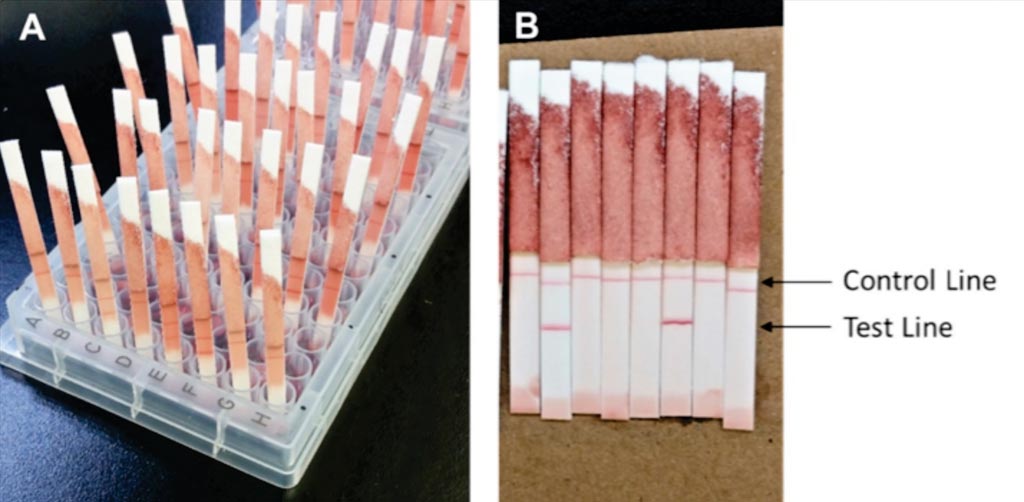
Image: A Pgp3 LFA-dipstick test. After approximately 4 hours, the LFA-dipstick was inserted into the well for 15 to 20 minutes, and then LFA buffer was added (A). LFAs were read when the membrane was completely cleared of background staining (B) (Photo courtesy of the CDC).
Trachoma, resulting from repeated infection with the bacterium Chlamydia trachomatis (Ct), is the leading infectious cause of blindness. Trachoma is targeted for elimination as a public health problem by 2020.
Serological surveillance for trachoma could allow monitoring of transmission levels in areas that have achieved elimination targets. Platforms that allow testing in basic laboratories or testing of easy-to-manage samples such as dried blood spots would contribute to the feasibility of serologic testing.
An international team of scientists led by the Centers for Disease Control and Prevention (Atlanta, GA, USA) collected blood from 506 1 to 12-year-olds in two villages in Kongwa district, Tanzania. The blood was tested for antibodies against the antigen Pgp3. Whole blood, plasma, and dried blood spots (DBS) were tested in laboratory and field settings using a cassette-enclosed Pgp3 lateral flow assay (LFA-cassette) and a pared-back “dipstick” assay (LFA-dipstick). DBS were also tested with a bead-based multiplex assay (MBA). Pooled ocular swab testing for Ct infection was performed using the GeneXpert CT/NG cartridge.
In the field evaluation of LFA-cassette using whole blood, the percent positive was 42.5% and the sensitivity was 84.2%. In the field evaluation of LFA-cassette using plasma, the percent positive was 48.4% and the sensitivity was 94.7%. The percent agreement between LFA-cassette and LFA-dipstick was 93.8%. The percent agreement between the MBA and each LFA format and sample type ranged from 92.3% to 94.7%. The percent positive by MBA was 45.5%, and the sensitivity was 94.7%. Interrater agreement between an expert rater and three different raters in field and laboratory settings was uniformly good.
The authors concluded that despite the minor differences in individual tests run, there were no significant differences in the percentage of positive samples as determined by MBA or any LFA tests. The data suggest that the LFA, in any number of iterations, and MBA could potentially be used interchangeably in a population-level, programmatic setting based on the program needs. The study was published in the April 2019 issue of the journal Diagnostic Microbiology and Infectious Disease.
Related Links:
Centers for Disease Control and Prevention
Serological surveillance for trachoma could allow monitoring of transmission levels in areas that have achieved elimination targets. Platforms that allow testing in basic laboratories or testing of easy-to-manage samples such as dried blood spots would contribute to the feasibility of serologic testing.
An international team of scientists led by the Centers for Disease Control and Prevention (Atlanta, GA, USA) collected blood from 506 1 to 12-year-olds in two villages in Kongwa district, Tanzania. The blood was tested for antibodies against the antigen Pgp3. Whole blood, plasma, and dried blood spots (DBS) were tested in laboratory and field settings using a cassette-enclosed Pgp3 lateral flow assay (LFA-cassette) and a pared-back “dipstick” assay (LFA-dipstick). DBS were also tested with a bead-based multiplex assay (MBA). Pooled ocular swab testing for Ct infection was performed using the GeneXpert CT/NG cartridge.
In the field evaluation of LFA-cassette using whole blood, the percent positive was 42.5% and the sensitivity was 84.2%. In the field evaluation of LFA-cassette using plasma, the percent positive was 48.4% and the sensitivity was 94.7%. The percent agreement between LFA-cassette and LFA-dipstick was 93.8%. The percent agreement between the MBA and each LFA format and sample type ranged from 92.3% to 94.7%. The percent positive by MBA was 45.5%, and the sensitivity was 94.7%. Interrater agreement between an expert rater and three different raters in field and laboratory settings was uniformly good.
The authors concluded that despite the minor differences in individual tests run, there were no significant differences in the percentage of positive samples as determined by MBA or any LFA tests. The data suggest that the LFA, in any number of iterations, and MBA could potentially be used interchangeably in a population-level, programmatic setting based on the program needs. The study was published in the April 2019 issue of the journal Diagnostic Microbiology and Infectious Disease.
Related Links:
Centers for Disease Control and Prevention
Latest Microbiology News
- New CE-Marked Hepatitis Assays to Help Diagnose Infections Earlier
- 1 Hour, Direct-From-Blood Multiplex PCR Test Identifies 95% of Sepsis-Causing Pathogens
- Mouth Bacteria Test Could Predict Colon Cancer Progression
- Unique Metabolic Signature Could Enable Sepsis Diagnosis within One Hour of Blood Collection
- Groundbreaking Diagnostic Platform Provides AST Results With Unprecedented Speed
- Simple Blood Test Combined With Personalized Risk Model Improves Sepsis Diagnosis
- Blood Analysis Predicts Sepsis and Organ Failure in Children
- TB Blood Test Could Detect Millions of Silent Spreaders
- New Blood Test Cuts Diagnosis Time for Nontuberculous Mycobacteria Infections from Months to Hours
- New Tuberculosis Test to Expand Testing Access in Low- and Middle-Income Countries
- Rapid Test Diagnoses Tropical Disease within Hours for Faster Antibiotics Treatment
- Rapid Molecular Testing Enables Faster, More Targeted Antibiotic Treatment for Pneumonia
- Rapid AST Platform Provides Targeted Therapeutic Results Days Faster Than Current Standard of Care
- New Analysis Method Detects Pathogens in Blood Faster and More Accurately by Melting DNA
- Rapid Sepsis Test Delivers Two Days Faster Results
- Portable Rapid PCR Diagnostic to Detect Gonorrhea and Antibiotic Susceptibility
Channels
Clinical Chemistry
view channel
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more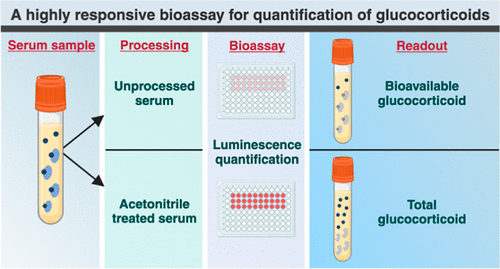
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channel
Blood Test Accurately Predicts Lung Cancer Risk and Reduces Need for Scans
Lung cancer is extremely hard to detect early due to the limitations of current screening technologies, which are costly, sometimes inaccurate, and less commonly endorsed by healthcare professionals compared... Read more
Unique Autoantibody Signature to Help Diagnose Multiple Sclerosis Years before Symptom Onset
Autoimmune diseases such as multiple sclerosis (MS) are thought to occur partly due to unusual immune responses to common infections. Early MS symptoms, including dizziness, spasms, and fatigue, often... Read more
Blood Test Could Detect HPV-Associated Cancers 10 Years before Clinical Diagnosis
Human papilloma virus (HPV) is known to cause various cancers, including those of the genitals, anus, mouth, throat, and cervix. HPV-associated oropharyngeal cancer (HPV+OPSCC) is the most common HPV-associated... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read morePathology
view channelAI-Powered Digital Imaging System to Revolutionize Cancer Diagnosis
The process of biopsy is important for confirming the presence of cancer. In the conventional histopathology technique, tissue is excised, sliced, stained, mounted on slides, and examined under a microscope... Read more
New Mycobacterium Tuberculosis Panel to Support Real-Time Surveillance and Combat Antimicrobial Resistance
Tuberculosis (TB), the leading cause of death from an infectious disease globally, is a contagious bacterial infection that primarily spreads through the coughing of patients with active pulmonary TB.... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more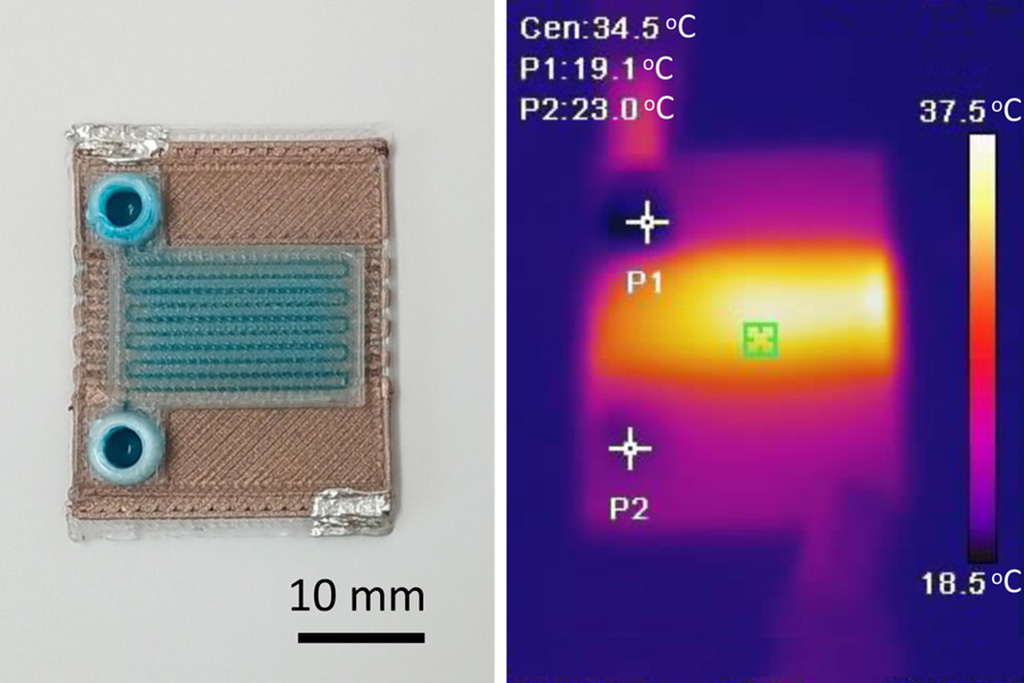
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel
ECCMID Congress Name Changes to ESCMID Global
Over the last few years, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID, Basel, Switzerland) has evolved remarkably. The society is now stronger and broader than ever before... Read more
Bosch and Randox Partner to Make Strategic Investment in Vivalytic Analysis Platform
Given the presence of so many diseases, determining whether a patient is presenting the symptoms of a simple cold, the flu, or something as severe as life-threatening meningitis is usually only possible... Read more
Siemens to Close Fast Track Diagnostics Business
Siemens Healthineers (Erlangen, Germany) has announced its intention to close its Fast Track Diagnostics unit, a small collection of polymerase chain reaction (PCR) testing products that is part of the... Read more