Danaher Acquires Integrated DNA Technologies
|
By LabMedica International staff writers Posted on 13 Mar 2018 |
Danaher Corporation (Washington, D.C, USA) has entered into a definitive agreement to acquire Integrated DNA Technologies (IDT) (Skokie, IL, USA), a privately held provider of high-value consumables for genomics applications in molecular biology, qPCR, next generation sequencing, synthetic biology, gene editing and molecular diagnostics.
IDT develops, manufactures, and markets nucleic acid products for the life sciences industry in the areas of academic research, biotechnology, agriculture, medical diagnostics, and pharmaceutical development. The company's primary business is the production of custom oligonucleotides for molecular biology applications. IDT has developed proprietary technologies for genomics applications such as next generation sequencing, CRISPR genome editing, qPCR, and RNA interference. Through its GMP services, IDT manufactures products used in diagnostic tests for many forms of cancer and most inherited and infectious diseases. IDT will operate as a standalone operating company and brand within Danaher's Life Sciences platform.
"We are thrilled to have IDT join Danaher's Life Sciences platform," said Rainer Blair, Executive Vice President of Danaher's Life Sciences platform. "IDT expands our presence into the highly attractive genomics market and will help play a central role in accelerating our customers' research and time to market as they develop critical diagnostic tests and potential life-saving therapies. IDT's historical double-digit core revenue growth and strong margins are a testament to the team's commitment to the highest standards of quality, service, and technical expertise."
"Danaher is a proven steward of founder-led businesses, such as Hach and Phenomenex. We recognize the extraordinary contributions Dr. Walder and IDT have made to the advancement of genomics research and applications over the past 30 years," said Thomas P. Joyce, Jr., Danaher President and Chief Executive Officer. "We look forward to supporting the IDT team and helping them leverage the tools of the Danaher Business System (DBS) to further enhance their growth profile and continue to create long-term customer value."
"For more than 30 years, IDT's innovative tools and solutions for genomics applications have helped scientists advance their research and contribute to solving some of the world's most vexing diseases and other challenges addressed by the life sciences community," added Joseph Walder, CEO and Chairman of IDT. "Joining Danaher will allow us to accelerate the high pace of innovation and superior service our customers have come to expect from us, as well as help expand our global reach. I'm excited to watch IDT further grow and innovate in this expanding area of genomics with the help of DBS."
IDT develops, manufactures, and markets nucleic acid products for the life sciences industry in the areas of academic research, biotechnology, agriculture, medical diagnostics, and pharmaceutical development. The company's primary business is the production of custom oligonucleotides for molecular biology applications. IDT has developed proprietary technologies for genomics applications such as next generation sequencing, CRISPR genome editing, qPCR, and RNA interference. Through its GMP services, IDT manufactures products used in diagnostic tests for many forms of cancer and most inherited and infectious diseases. IDT will operate as a standalone operating company and brand within Danaher's Life Sciences platform.
"We are thrilled to have IDT join Danaher's Life Sciences platform," said Rainer Blair, Executive Vice President of Danaher's Life Sciences platform. "IDT expands our presence into the highly attractive genomics market and will help play a central role in accelerating our customers' research and time to market as they develop critical diagnostic tests and potential life-saving therapies. IDT's historical double-digit core revenue growth and strong margins are a testament to the team's commitment to the highest standards of quality, service, and technical expertise."
"Danaher is a proven steward of founder-led businesses, such as Hach and Phenomenex. We recognize the extraordinary contributions Dr. Walder and IDT have made to the advancement of genomics research and applications over the past 30 years," said Thomas P. Joyce, Jr., Danaher President and Chief Executive Officer. "We look forward to supporting the IDT team and helping them leverage the tools of the Danaher Business System (DBS) to further enhance their growth profile and continue to create long-term customer value."
"For more than 30 years, IDT's innovative tools and solutions for genomics applications have helped scientists advance their research and contribute to solving some of the world's most vexing diseases and other challenges addressed by the life sciences community," added Joseph Walder, CEO and Chairman of IDT. "Joining Danaher will allow us to accelerate the high pace of innovation and superior service our customers have come to expect from us, as well as help expand our global reach. I'm excited to watch IDT further grow and innovate in this expanding area of genomics with the help of DBS."
Latest Industry News
- Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
- Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
- Thermo Fisher and Bio-Techne Enter Into Strategic Distribution Agreement for Europe
- ECCMID Congress Name Changes to ESCMID Global
- Bosch and Randox Partner to Make Strategic Investment in Vivalytic Analysis Platform
- Siemens to Close Fast Track Diagnostics Business
- Beckman Coulter and Fujirebio Expand Partnership on Neurodegenerative Disease Diagnostics
- Sysmex and Hitachi Collaborate on Development of New Genetic Testing Systems
- Sysmex and CellaVision Expand Collaboration to Advance Hematology Solutions
- BD and Techcyte Collaborate on AI-Based Digital Cervical Cytology System for Pap Testing
- Medlab Middle East 2024 to Address Transformative Potential of Artificial Intelligence
- Seegene and Microsoft Collaborate to Realize a World Free from All Diseases and Future Pandemics
- Medlab Middle East 2024 to Highlight Importance of Sustainability in Laboratories
- Fujirebio and Agappe Collaborate on CLIA-Based Immunoassay
- Medlab Middle East 2024 to Highlight Groundbreaking NextGen Medicine
- bioMérieux Acquires Software Company LUMED to Support Fight against Antimicrobial Resistance
Channels
Clinical Chemistry
view channel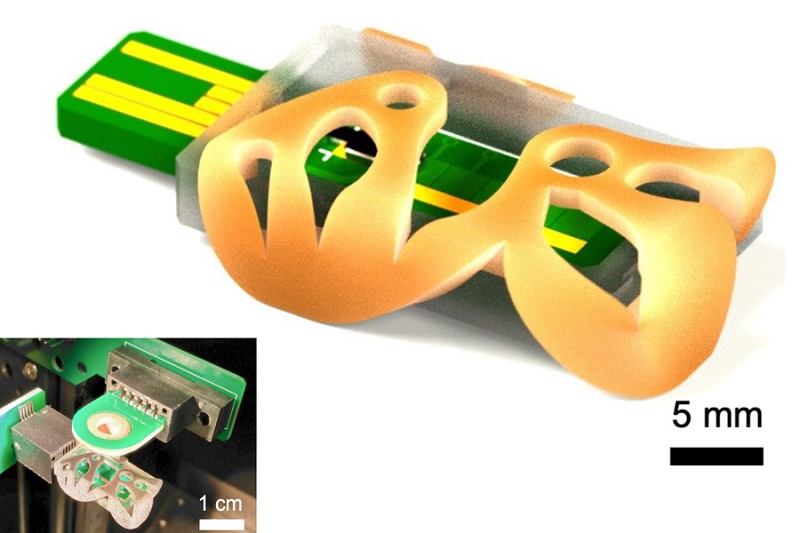
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more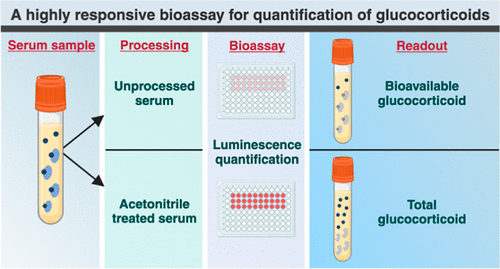
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channel
Novel Biomarkers to Improve Diagnosis of Renal Cell Carcinoma Subtypes
Renal cell carcinomas (RCCs) are notably diverse, encompassing over 20 distinct subtypes and generally categorized into clear cell and non-clear cell types; around 20% of all RCCs fall into the non-clear... Read more
RNA-Powered Molecular Test to Help Combat Early-Age Onset Colorectal Cancer
Colorectal cancer (CRC) ranks as the second most lethal cancer in the United States. Nevertheless, many Americans eligible for screening do not undergo testing due to limited access or reluctance towards... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more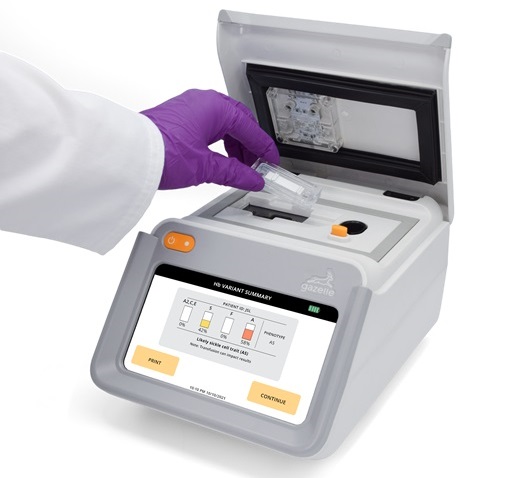
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read moreMicrobiology
view channel
Integrated Solution Ushers New Era of Automated Tuberculosis Testing
Tuberculosis (TB) is responsible for 1.3 million deaths every year, positioning it as one of the top killers globally due to a single infectious agent. In 2022, around 10.6 million people were diagnosed... Read more
Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
Sepsis affects up to 50 million people globally each year, with bacteraemia, formerly known as blood poisoning, being a major cause. In the United States alone, approximately two million individuals are... Read moreEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read morePathology
view channelHyperspectral Dark-Field Microscopy Enables Rapid and Accurate Identification of Cancerous Tissues
Breast cancer remains a major cause of cancer-related mortality among women. Breast-conserving surgery (BCS), also known as lumpectomy, is the removal of the cancerous lump and a small margin of surrounding tissue.... Read more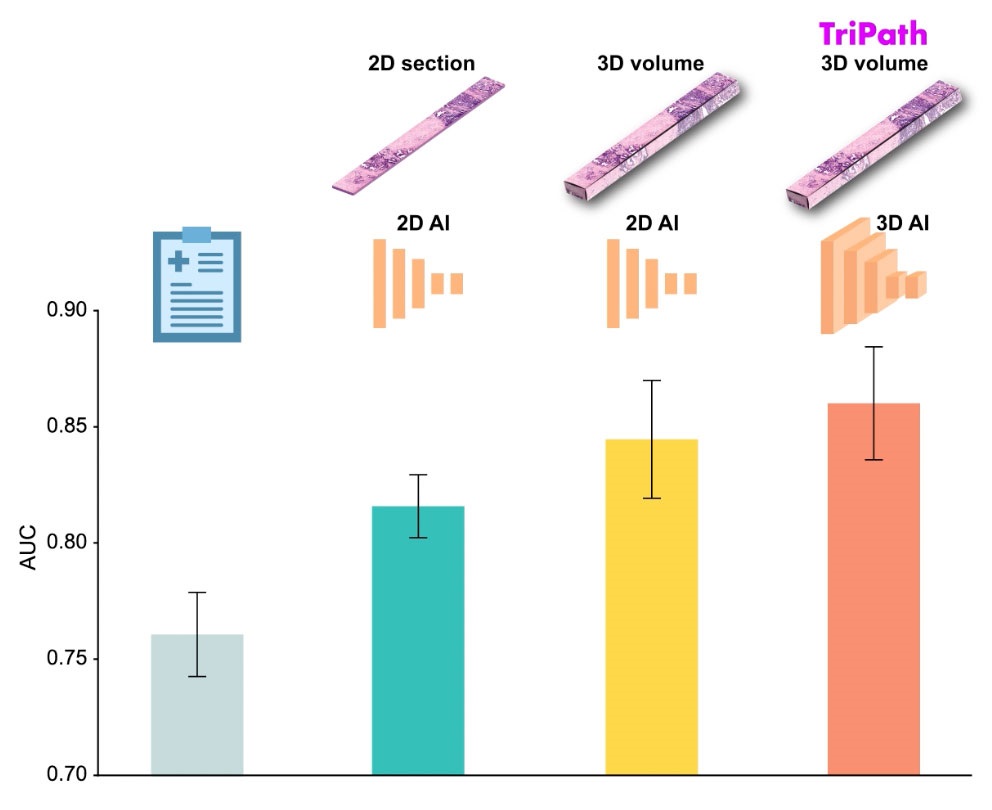
AI Advancements Enable Leap into 3D Pathology
Human tissue is complex, intricate, and naturally three-dimensional. However, the thin two-dimensional tissue slices commonly used by pathologists to diagnose diseases provide only a limited view of the... Read more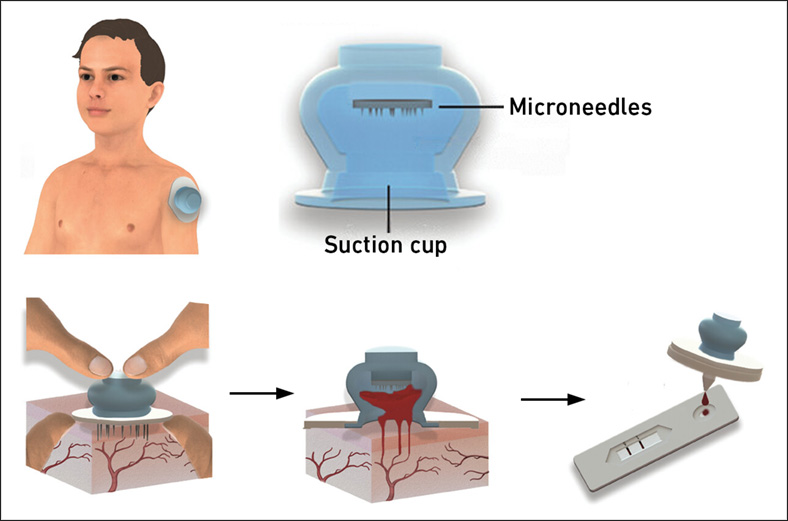
New Blood Test Device Modeled on Leeches to Help Diagnose Malaria
Many individuals have a fear of needles, making the experience of having blood drawn from their arm particularly distressing. An alternative method involves taking blood from the fingertip or earlobe,... Read more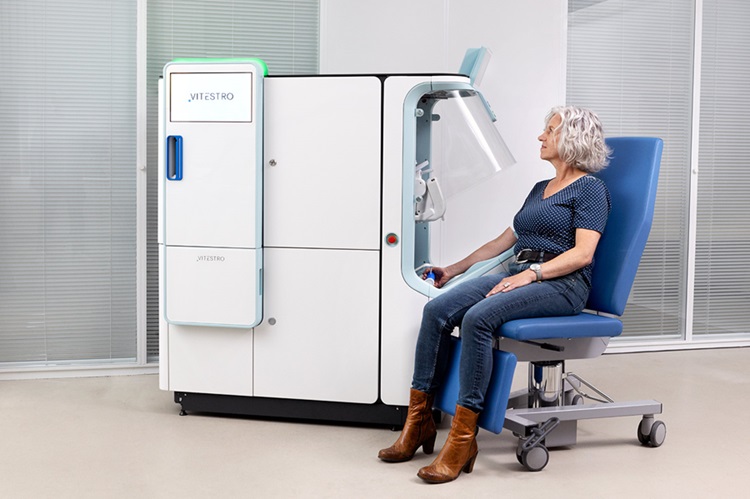
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages,... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more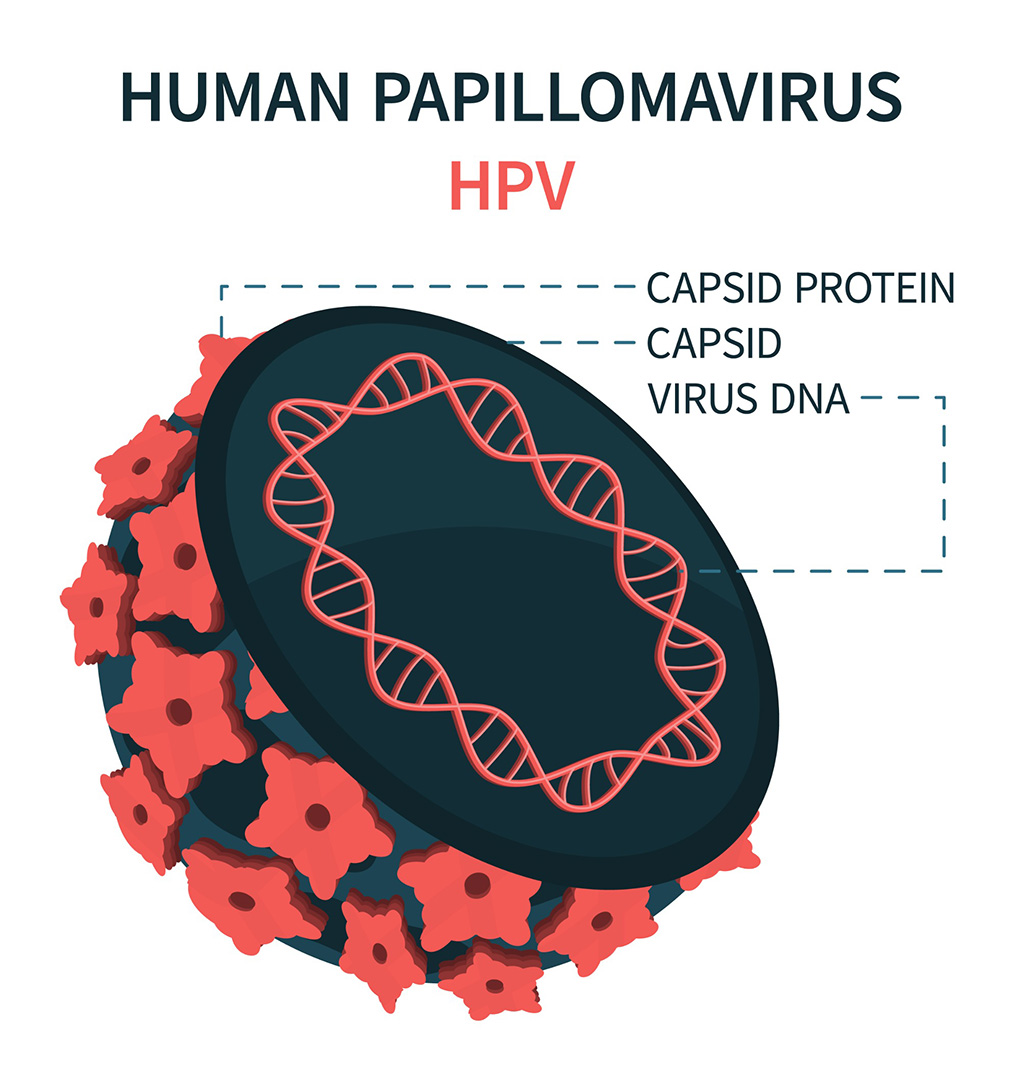
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more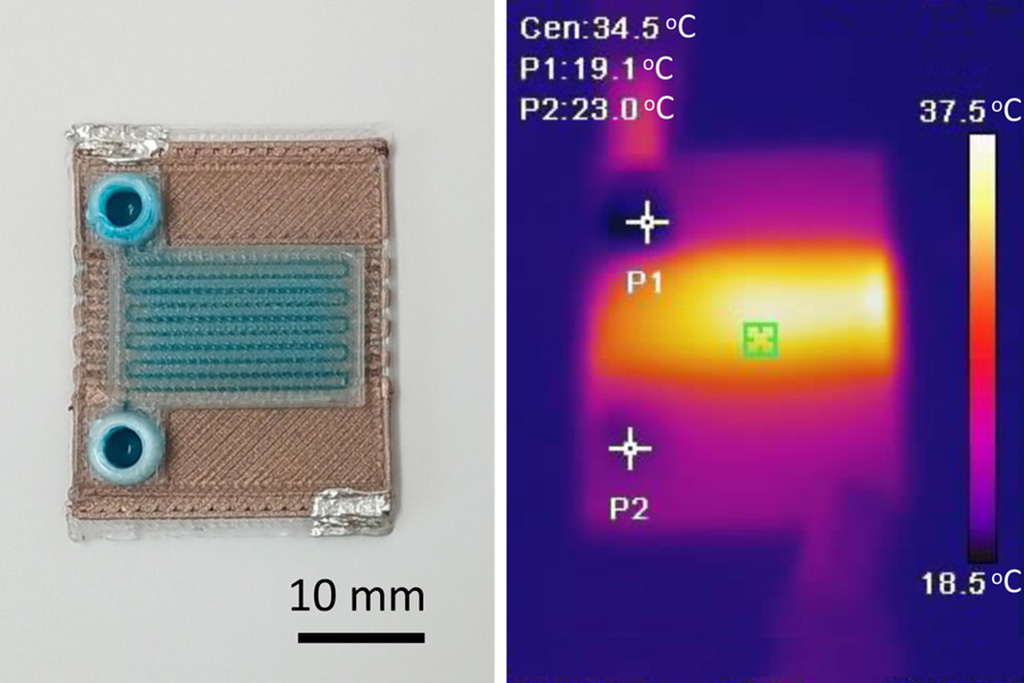
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more