Immunoassay Biomarkers Evaluated for Preeclampsia Diagnosis
|
By LabMedica International staff writers Posted on 17 Aug 2017 |

Image: The Brahms Kryptor automated immunoassay platform (Photo courtesy of Thermo Fisher Scientific).
Preeclampsia is one of the leading hypertensive disorders in pregnant women worldwide and it is estimated to cause more than 60,000 deaths per year. Preeclampsia is induced by placental and maternal vascular dysfunction, which affects both maternal and neonatal health.
The current diagnosis of preeclampsia is based on non-specific tests such as new onset of hypertension and proteinuria after 20 weeks of gestation. More recently, two emerging biomarkers, anti-angiogenic factor soluble fms-like tyrosine kinase 1 (sFlt-1) and pro-angiogenic factor placental growth factor (PlGF), have been demonstrated to be involved in the pathophysiology of preeclampsia.
Scientists at the University of Chicago, Chicago, IL, USA) and their colleagues examined K2-EDTA plasma samples from 50 patients on, an automated immunoassay platform. Quality control (QC) materials were used to access intra- and inter-precision of the assay. Lower limit of quantitation and interference studies were determined using pooled patient plasma. Studies have shown that the usefulness of sFlt-1/PlGF ratio in confirming or ruling out suspected preeclampsia cases. Thus wider availability of the automated measurement of sFlt-1 and PlGF has the potential of improving the clinical management of preeclampsia.
The team used the Brahms Kryptor automated immunoassay platform (BRAHMS, Thermo Fisher Scientific, Waltham, MA, USA) and demonstrated an analytical measuring range for sFlt-1 and PlGF assays of 90-69,000 pg/mL and 11-7,000 pg/mL, respectively. The lower limit of quantitation (20% CV) was interpolated to be 35 pg/mL for sFlt-1 and 10 pg/mL for PlGF. Total precision for both assay displayed CVs of less than 10%. Interference studies showed that both assays were not significantly affected by hemolysis up to an H-index of 1100 for sFlt-1 and 300 for PlGF; L- and I- index of 800 and 80 respectively for both assays. When compared to another institute’s method the larger differences in overall bias based on individual tests however was greatly improved when a ratio of sFlt/PlGF is taken into account for method comparison.
The authors concluded that the pre-eclamptic biomarkers, sFlt-1 and PlGF assayed on the Brahms Kryptor platform, demonstrate good analytical performance and are acceptable for clinical studies defining the role of these biomarkers for preeclampsia. The study was presented at the 69th Annual meeting of American Association for Clinical Chemistry held August 1–3, 2017, in San Diego, CA, USA.
The current diagnosis of preeclampsia is based on non-specific tests such as new onset of hypertension and proteinuria after 20 weeks of gestation. More recently, two emerging biomarkers, anti-angiogenic factor soluble fms-like tyrosine kinase 1 (sFlt-1) and pro-angiogenic factor placental growth factor (PlGF), have been demonstrated to be involved in the pathophysiology of preeclampsia.
Scientists at the University of Chicago, Chicago, IL, USA) and their colleagues examined K2-EDTA plasma samples from 50 patients on, an automated immunoassay platform. Quality control (QC) materials were used to access intra- and inter-precision of the assay. Lower limit of quantitation and interference studies were determined using pooled patient plasma. Studies have shown that the usefulness of sFlt-1/PlGF ratio in confirming or ruling out suspected preeclampsia cases. Thus wider availability of the automated measurement of sFlt-1 and PlGF has the potential of improving the clinical management of preeclampsia.
The team used the Brahms Kryptor automated immunoassay platform (BRAHMS, Thermo Fisher Scientific, Waltham, MA, USA) and demonstrated an analytical measuring range for sFlt-1 and PlGF assays of 90-69,000 pg/mL and 11-7,000 pg/mL, respectively. The lower limit of quantitation (20% CV) was interpolated to be 35 pg/mL for sFlt-1 and 10 pg/mL for PlGF. Total precision for both assay displayed CVs of less than 10%. Interference studies showed that both assays were not significantly affected by hemolysis up to an H-index of 1100 for sFlt-1 and 300 for PlGF; L- and I- index of 800 and 80 respectively for both assays. When compared to another institute’s method the larger differences in overall bias based on individual tests however was greatly improved when a ratio of sFlt/PlGF is taken into account for method comparison.
The authors concluded that the pre-eclamptic biomarkers, sFlt-1 and PlGF assayed on the Brahms Kryptor platform, demonstrate good analytical performance and are acceptable for clinical studies defining the role of these biomarkers for preeclampsia. The study was presented at the 69th Annual meeting of American Association for Clinical Chemistry held August 1–3, 2017, in San Diego, CA, USA.
Latest Immunology News
- Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
- AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
- Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
- Testing Method Could Help More Patients Receive Right Cancer Treatment
- Groundbreaking Test Monitors Radiation Therapy Toxicity in Cancer Patients
- State-Of-The Art Techniques to Investigate Immune Response in Deadly Strep A Infections
- Novel Immunoassays Enable Early Diagnosis of Antiphospholipid Syndrome
- New Test Could Predict Immunotherapy Success for Broader Range Of Cancers
- Simple Blood Protein Tests Predict CAR T Outcomes for Lymphoma Patients
- Cell Sorter Chip Technology to Pave Way for Immune Profiling at POC
- Chip Monitors Cancer Cells in Blood Samples to Assess Treatment Effectiveness
- Automated Immunohematology Approaches Can Resolve Transplant Incompatibility
- AI Leverages Tumor Genetics to Predict Patient Response to Chemotherapy
- World’s First Portable, Non-Invasive WBC Monitoring Device to Eliminate Need for Blood Draw
- Predictive T-Cell Test Detects Immune Response to Viruses Even Before Antibodies Form
- Single Blood Draw to Detect Immune Cells Present Months before Flu Infection Can Predict Symptoms
Channels
Clinical Chemistry
view channel
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more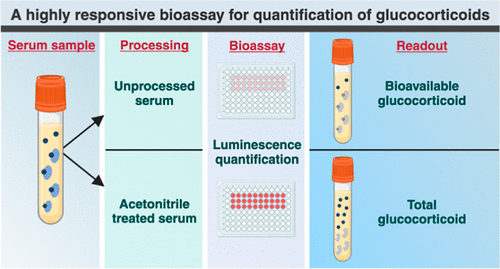
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channel
Urine Test to Revolutionize Lyme Disease Testing
Lyme disease is the most common animal-to-human transmitted disease in the United States, with around 476,000 people diagnosed and treated annually, and its incidence has been increasing.... Read more
Simple Blood Test Could Enable First Quantitative Assessments for Future Cerebrovascular Disease
Cerebral small vessel disease is a common cause of stroke and cognitive decline, particularly in the elderly. Presently, assessing the risk for cerebral vascular diseases involves using a mix of diagnostic... Read more
New Genetic Testing Procedure Combined With Ultrasound Detects High Cardiovascular Risk
A key interest area in cardiovascular research today is the impact of clonal hematopoiesis on cardiovascular diseases. Clonal hematopoiesis results from mutations in hematopoietic stem cells and may lead... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreMicrobiology
view channelEnhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
GenMark Diagnostics (Carlsbad, CA, USA), a member of the Roche Group (Basel, Switzerland), has rebranded its ePlex® system as the cobas eplex system. This rebranding under the globally renowned cobas name... Read more
Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
Antimicrobial resistance (AMR) is a serious global public health concern that claims millions of lives every year. It primarily results from the inappropriate and excessive use of antibiotics, which reduces... Read more
New CE-Marked Hepatitis Assays to Help Diagnose Infections Earlier
According to the World Health Organization (WHO), an estimated 354 million individuals globally are afflicted with chronic hepatitis B or C. These viruses are the leading causes of liver cirrhosis, liver... Read more
1 Hour, Direct-From-Blood Multiplex PCR Test Identifies 95% of Sepsis-Causing Pathogens
Sepsis contributes to one in every three hospital deaths in the US, and globally, septic shock carries a mortality rate of 30-40%. Diagnosing sepsis early is challenging due to its non-specific symptoms... Read morePathology
view channel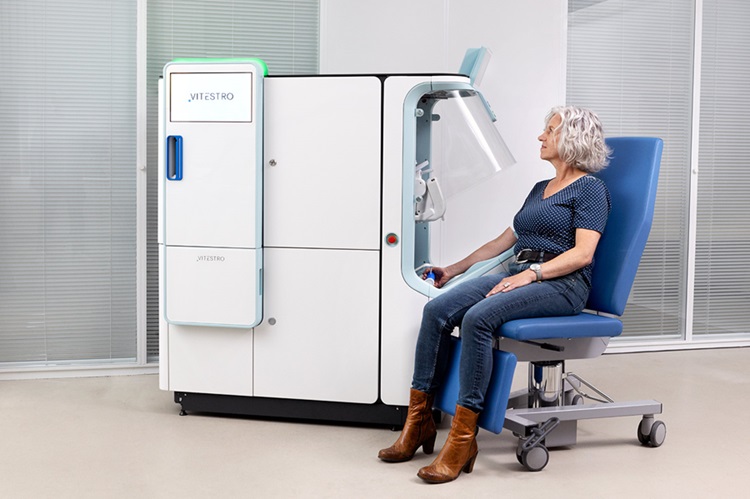
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages,... Read more.jpg)
Use of DICOM Images for Pathology Diagnostics Marks Significant Step towards Standardization
Digital pathology is rapidly becoming a key aspect of modern healthcare, transforming the practice of pathology as laboratories worldwide adopt this advanced technology. Digital pathology systems allow... Read more
First of Its Kind Universal Tool to Revolutionize Sample Collection for Diagnostic Tests
The COVID pandemic has dramatically reshaped the perception of diagnostics. Post the pandemic, a groundbreaking device that combines sample collection and processing into a single, easy-to-use disposable... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more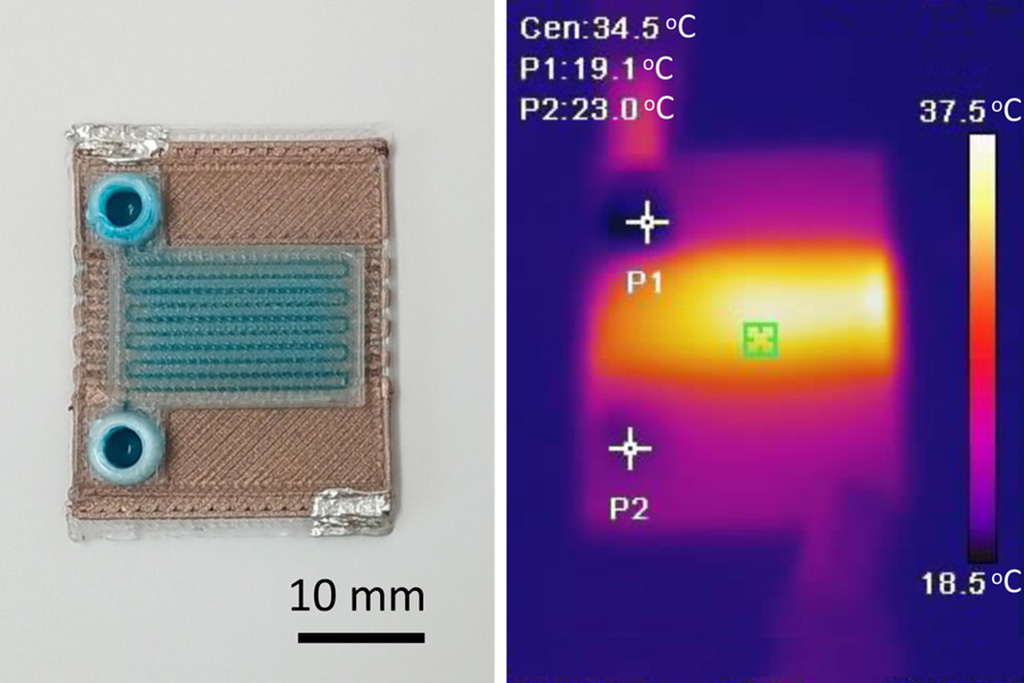
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel_1.jpg)
Thermo Fisher and Bio-Techne Enter Into Strategic Distribution Agreement for Europe
Thermo Fisher Scientific (Waltham, MA USA) has entered into a strategic distribution agreement with Bio-Techne Corporation (Minneapolis, MN, USA), resulting in a significant collaboration between two industry... Read more
ECCMID Congress Name Changes to ESCMID Global
Over the last few years, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID, Basel, Switzerland) has evolved remarkably. The society is now stronger and broader than ever before... Read more