Immunochromatography Tests Evaluated for Detection of Novel Norovirus
|
By LabMedica International staff writers Posted on 02 Aug 2015 |
Image: Transmission electron micrograph (TEM) of norovirus virions, or virus particles (Photo courtesy of Charles D. Humphrey/CDC).
A novel GII.17 Norovirus has emerged as a major cause of epidemic and endemic acute gastroenteritis in several countries in Asia.
From September 2014 to March 2015, 70% of all outbreaks in Guangdong and Jiangsu provinces in China were caused by a novel GII.17 virus and a similar increase in the number of infections with this novel GII.17 virus has been reported in Japan and Thailand since December 2014.
Microbiologists at Chiang Mai University (Thailand) working with colleagues from Japan, analyzed randomly selected stool samples for which there was a large quantity available—a panel of six GII.17-positive stool samples from five patients in Japan and one in Thailand in which the virus copy numbers had been quantified by real-time reverse-transcriptase polymerase chain reaction (RT-PC R) for reference values. Two of the six GII.17 stool samples tested positive by four immunochromatography (IC) kits.
To evaluate if these IC tests are able to detect these novel GII.17 noroviruses, they tested four commercial kits available in Japan: GE test Noro Nissui (Nissui Pharmaceutical Co., Ltd.; Tokyo, Japan); ImmunoCatch-Noro (Eiken Chemical Co., Ltd.; Tokyo, Japan); Quick Navi-Noro 2 (Denka Seiken Co., Ltd.; Tokyo, Japan); Quick Chaser-Noro (Mizuho Medy Co., Ltd.; Tosu City, Japan).
The six specimens tested by real-time RT-PCR demonstrated that the two samples that were positive by IC test contained high virus titers of 1.90 × 109 and 8.06 × 109 virus copies/mL. In contrast, the other four specimens that were negative in the IC test had virus titers ranging from 4.91 × 103 to 2.50 ×108 virus copies/mL. The results demonstrated that at 1:10 dilution of a virus titer equal to 1.90 × 108 copies/mL, two of three tests still showed the positive results, with a weak positive band, while at 1:100 dilution equal to virus titer 1.90 × 107 copies/mL, all three IC tests were negative. These data demonstrated that the sensitivity of the IC kits for the detection of this novel GII.17 virus was about 108 copies/mL.
The authors concluded that the tested commercial IC kits for the detection Norovirus available on the market in Japan were able to detect the novel GII.17 Norovirus, but with relatively low sensitivity. Only samples that contained more than 109 copies/mL were positive in all four IC tests. They suggest a redesign of the currently available Norovirus IC tests may be required to detect the novel GII.17 noroviruses with the same sensitivity as for the more commonly circulating norovirus genotypes. Laboratories and physicians should be aware of these findings, in particular where the novel GII.17 norovirus has been shown to be circulating.
Related Links:
Chiang Mai University
Nissui Pharmaceutical
Eiken Chemical
From September 2014 to March 2015, 70% of all outbreaks in Guangdong and Jiangsu provinces in China were caused by a novel GII.17 virus and a similar increase in the number of infections with this novel GII.17 virus has been reported in Japan and Thailand since December 2014.
Microbiologists at Chiang Mai University (Thailand) working with colleagues from Japan, analyzed randomly selected stool samples for which there was a large quantity available—a panel of six GII.17-positive stool samples from five patients in Japan and one in Thailand in which the virus copy numbers had been quantified by real-time reverse-transcriptase polymerase chain reaction (RT-PC R) for reference values. Two of the six GII.17 stool samples tested positive by four immunochromatography (IC) kits.
To evaluate if these IC tests are able to detect these novel GII.17 noroviruses, they tested four commercial kits available in Japan: GE test Noro Nissui (Nissui Pharmaceutical Co., Ltd.; Tokyo, Japan); ImmunoCatch-Noro (Eiken Chemical Co., Ltd.; Tokyo, Japan); Quick Navi-Noro 2 (Denka Seiken Co., Ltd.; Tokyo, Japan); Quick Chaser-Noro (Mizuho Medy Co., Ltd.; Tosu City, Japan).
The six specimens tested by real-time RT-PCR demonstrated that the two samples that were positive by IC test contained high virus titers of 1.90 × 109 and 8.06 × 109 virus copies/mL. In contrast, the other four specimens that were negative in the IC test had virus titers ranging from 4.91 × 103 to 2.50 ×108 virus copies/mL. The results demonstrated that at 1:10 dilution of a virus titer equal to 1.90 × 108 copies/mL, two of three tests still showed the positive results, with a weak positive band, while at 1:100 dilution equal to virus titer 1.90 × 107 copies/mL, all three IC tests were negative. These data demonstrated that the sensitivity of the IC kits for the detection of this novel GII.17 virus was about 108 copies/mL.
The authors concluded that the tested commercial IC kits for the detection Norovirus available on the market in Japan were able to detect the novel GII.17 Norovirus, but with relatively low sensitivity. Only samples that contained more than 109 copies/mL were positive in all four IC tests. They suggest a redesign of the currently available Norovirus IC tests may be required to detect the novel GII.17 noroviruses with the same sensitivity as for the more commonly circulating norovirus genotypes. Laboratories and physicians should be aware of these findings, in particular where the novel GII.17 norovirus has been shown to be circulating.
Related Links:
Chiang Mai University
Nissui Pharmaceutical
Eiken Chemical
Latest Microbiology News
- Integrated Solution Ushers New Era of Automated Tuberculosis Testing
- Automated Sepsis Test System Enables Rapid Diagnosis for Patients with Severe Bloodstream Infections
- Enhanced Rapid Syndromic Molecular Diagnostic Solution Detects Broad Range of Infectious Diseases
- Clinical Decision Support Software a Game-Changer in Antimicrobial Resistance Battle
- New CE-Marked Hepatitis Assays to Help Diagnose Infections Earlier
- 1 Hour, Direct-From-Blood Multiplex PCR Test Identifies 95% of Sepsis-Causing Pathogens
- Mouth Bacteria Test Could Predict Colon Cancer Progression
- Unique Metabolic Signature Could Enable Sepsis Diagnosis within One Hour of Blood Collection
- Groundbreaking Diagnostic Platform Provides AST Results With Unprecedented Speed
- Simple Blood Test Combined With Personalized Risk Model Improves Sepsis Diagnosis
- Blood Analysis Predicts Sepsis and Organ Failure in Children
- TB Blood Test Could Detect Millions of Silent Spreaders
- New Blood Test Cuts Diagnosis Time for Nontuberculous Mycobacteria Infections from Months to Hours
- New Tuberculosis Test to Expand Testing Access in Low- and Middle-Income Countries
- Rapid Test Diagnoses Tropical Disease within Hours for Faster Antibiotics Treatment
- Rapid Molecular Testing Enables Faster, More Targeted Antibiotic Treatment for Pneumonia
Channels
Clinical Chemistry
view channel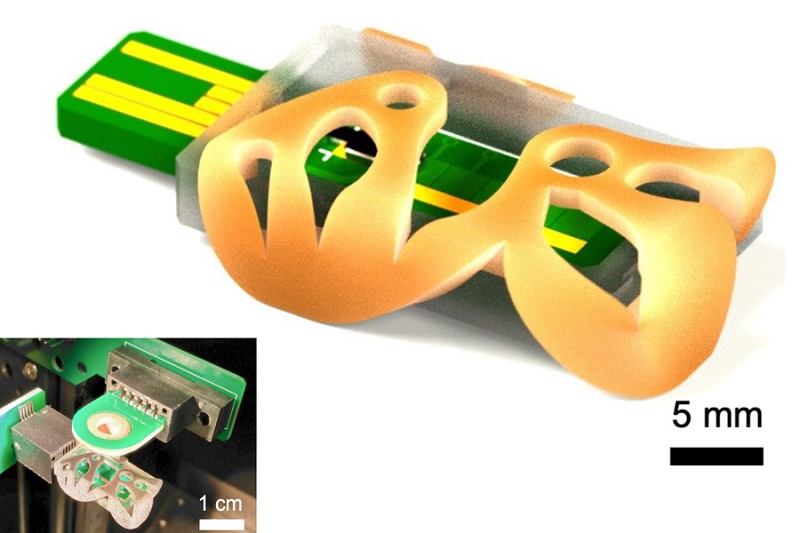
3D Printed Point-Of-Care Mass Spectrometer Outperforms State-Of-The-Art Models
Mass spectrometry is a precise technique for identifying the chemical components of a sample and has significant potential for monitoring chronic illness health states, such as measuring hormone levels... Read more.jpg)
POC Biomedical Test Spins Water Droplet Using Sound Waves for Cancer Detection
Exosomes, tiny cellular bioparticles carrying a specific set of proteins, lipids, and genetic materials, play a crucial role in cell communication and hold promise for non-invasive diagnostics.... Read more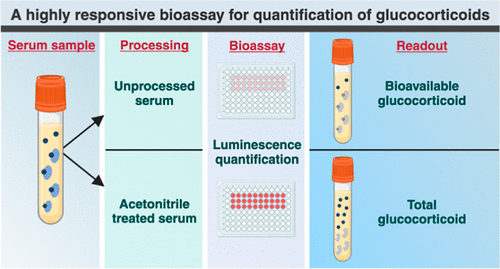
Highly Reliable Cell-Based Assay Enables Accurate Diagnosis of Endocrine Diseases
The conventional methods for measuring free cortisol, the body's stress hormone, from blood or saliva are quite demanding and require sample processing. The most common method, therefore, involves collecting... Read moreMolecular Diagnostics
view channelBlood Proteins Could Warn of Cancer Seven Years before Diagnosis
Two studies have identified proteins in the blood that could potentially alert individuals to the presence of cancer more than seven years before the disease is clinically diagnosed. Researchers found... Read moreUltrasound-Aided Blood Testing Detects Cancer Biomarkers from Cells
Ultrasound imaging serves as a noninvasive method to locate and monitor cancerous tumors effectively. However, crucial details about the cancer, such as the specific types of cells and genetic mutations... Read moreHematology
view channel
Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
Hemoglobinopathies, the most widespread inherited conditions globally, affect about 7% of the population as carriers, with 2.7% of newborns being born with these conditions. The spectrum of clinical manifestations... Read more
First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
Arboviruses represent an emerging global health threat, exacerbated by climate change and increased international travel that is facilitating their spread across new regions. Chikungunya, dengue, West... Read more
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
Neutropenia, a decrease in neutrophils (a type of white blood cell crucial for fighting infections), is a frequent side effect of certain cancer treatments. This condition elevates the risk of infections,... Read more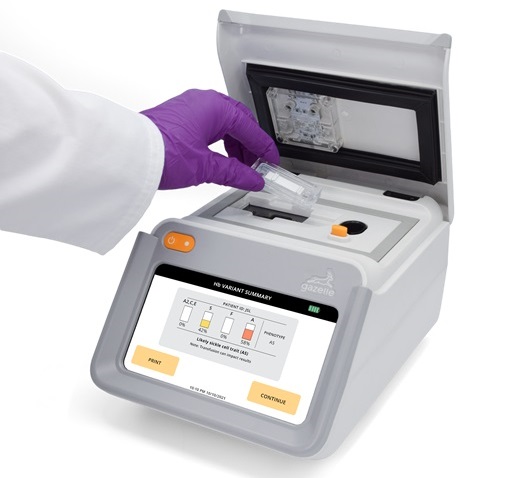
First Affordable and Rapid Test for Beta Thalassemia Demonstrates 99% Diagnostic Accuracy
Hemoglobin disorders rank as some of the most prevalent monogenic diseases globally. Among various hemoglobin disorders, beta thalassemia, a hereditary blood disorder, affects about 1.5% of the world's... Read moreImmunology
view channel.jpg)
AI Predicts Tumor-Killing Cells with High Accuracy
Cellular immunotherapy involves extracting immune cells from a patient's tumor, potentially enhancing their cancer-fighting capabilities through engineering, and then expanding and reintroducing them into the body.... Read more
Diagnostic Blood Test for Cellular Rejection after Organ Transplant Could Replace Surgical Biopsies
Transplanted organs constantly face the risk of being rejected by the recipient's immune system which differentiates self from non-self using T cells and B cells. T cells are commonly associated with acute... Read more
AI Tool Precisely Matches Cancer Drugs to Patients Using Information from Each Tumor Cell
Current strategies for matching cancer patients with specific treatments often depend on bulk sequencing of tumor DNA and RNA, which provides an average profile from all cells within a tumor sample.... Read more
Genetic Testing Combined With Personalized Drug Screening On Tumor Samples to Revolutionize Cancer Treatment
Cancer treatment typically adheres to a standard of care—established, statistically validated regimens that are effective for the majority of patients. However, the disease’s inherent variability means... Read morePathology
view channel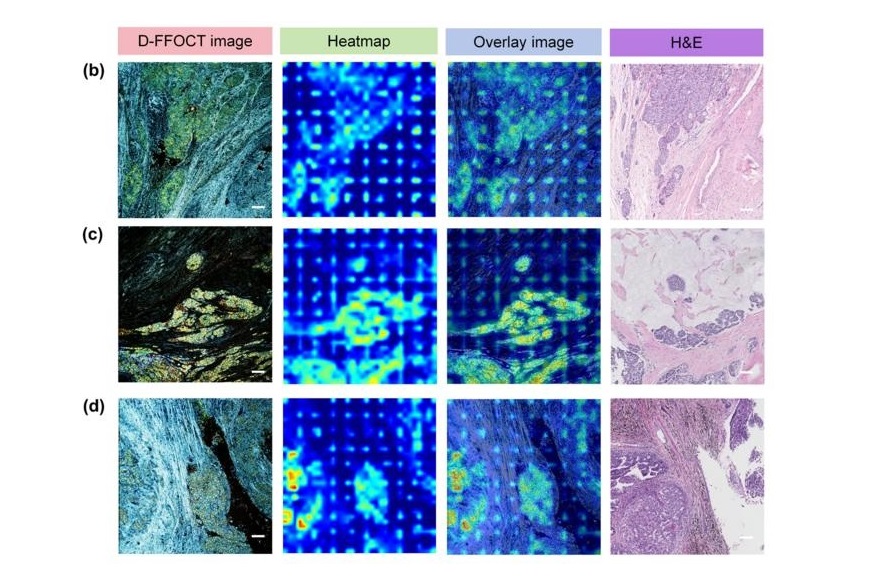
AI Integrated With Optical Imaging Technology Enables Rapid Intraoperative Diagnosis
Rapid and accurate intraoperative diagnosis is essential for tumor surgery as it guides surgical decisions with precision. Traditional intraoperative assessments, such as frozen sections based on H&E... Read more
HPV Self-Collection Solution Improves Access to Cervical Cancer Testing
Annually, over 604,000 women across the world are diagnosed with cervical cancer, and about 342,000 die from this disease, which is preventable and primarily caused by the Human Papillomavirus (HPV).... Read moreHyperspectral Dark-Field Microscopy Enables Rapid and Accurate Identification of Cancerous Tissues
Breast cancer remains a major cause of cancer-related mortality among women. Breast-conserving surgery (BCS), also known as lumpectomy, is the removal of the cancerous lump and a small margin of surrounding tissue.... Read moreTechnology
view channel
New Diagnostic System Achieves PCR Testing Accuracy
While PCR tests are the gold standard of accuracy for virology testing, they come with limitations such as complexity, the need for skilled lab operators, and longer result times. They also require complex... Read more
DNA Biosensor Enables Early Diagnosis of Cervical Cancer
Molybdenum disulfide (MoS2), recognized for its potential to form two-dimensional nanosheets like graphene, is a material that's increasingly catching the eye of the scientific community.... Read more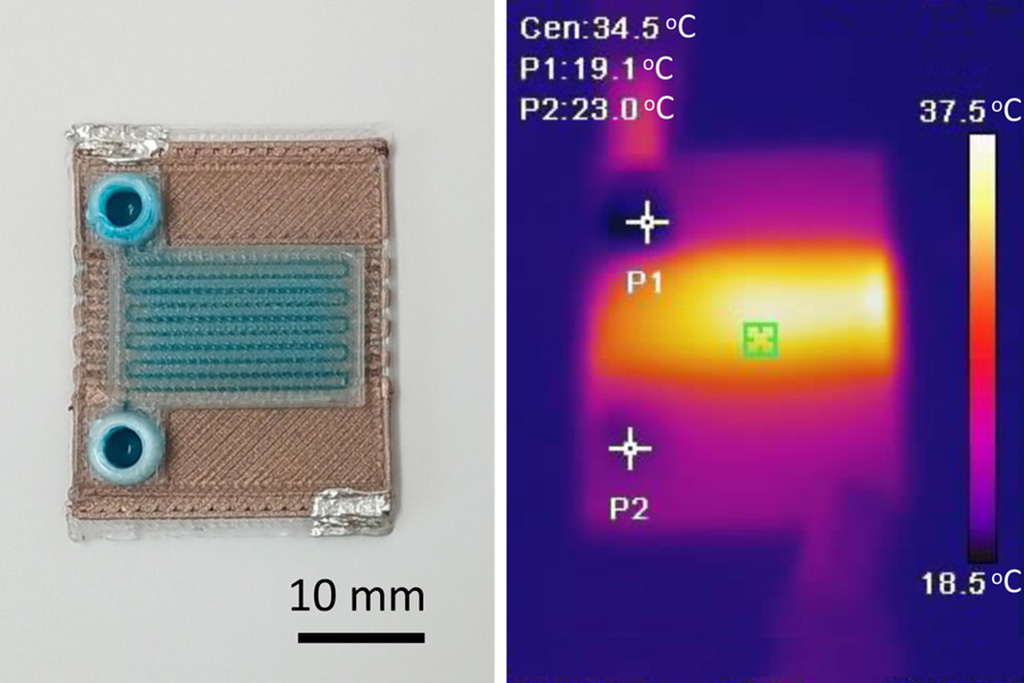
Self-Heating Microfluidic Devices Can Detect Diseases in Tiny Blood or Fluid Samples
Microfluidics, which are miniature devices that control the flow of liquids and facilitate chemical reactions, play a key role in disease detection from small samples of blood or other fluids.... Read more
Breakthrough in Diagnostic Technology Could Make On-The-Spot Testing Widely Accessible
Home testing gained significant importance during the COVID-19 pandemic, yet the availability of rapid tests is limited, and most of them can only drive one liquid across the strip, leading to continued... Read moreIndustry
view channel
Danaher and Johns Hopkins University Collaborate to Improve Neurological Diagnosis
Unlike severe traumatic brain injury (TBI), mild TBI often does not show clear correlations with abnormalities detected through head computed tomography (CT) scans. Consequently, there is a pressing need... Read more
Beckman Coulter and MeMed Expand Host Immune Response Diagnostics Partnership
Beckman Coulter Diagnostics (Brea, CA, USA) and MeMed BV (Haifa, Israel) have expanded their host immune response diagnostics partnership. Beckman Coulter is now an authorized distributor of the MeMed... Read more_1.jpg)












_1.jpg)
.jpg)