Siemens Begins Delivering CE-IVD Registered Coronavirus SARS-CoV-2 Test Kit
Siemens Healthineers (Erlangen, Germany) has registered its molecular Fast Track Diagnostics (FTD) SARS-CoV-2 Assay2 test kit for diagnostic use with the Luxembourg Ministry of Health, allowing for its immediate rollout for diagnostic use in Europe. More...30 Apr 2020
Molecular Diagnostics (MDx) And Lateral Flow Assays (LAFs) Dominate COVID-19 Diagnostics, Says New Report
The need for universal and massive testing across the population has led to a race for technology innovations for COVID-19 diagnostics. From the technological perspective, molecular diagnostics (MDx) and lateral flow assays (LAFs) dominate COVID-19 diagnostics. More...30 Apr 2020


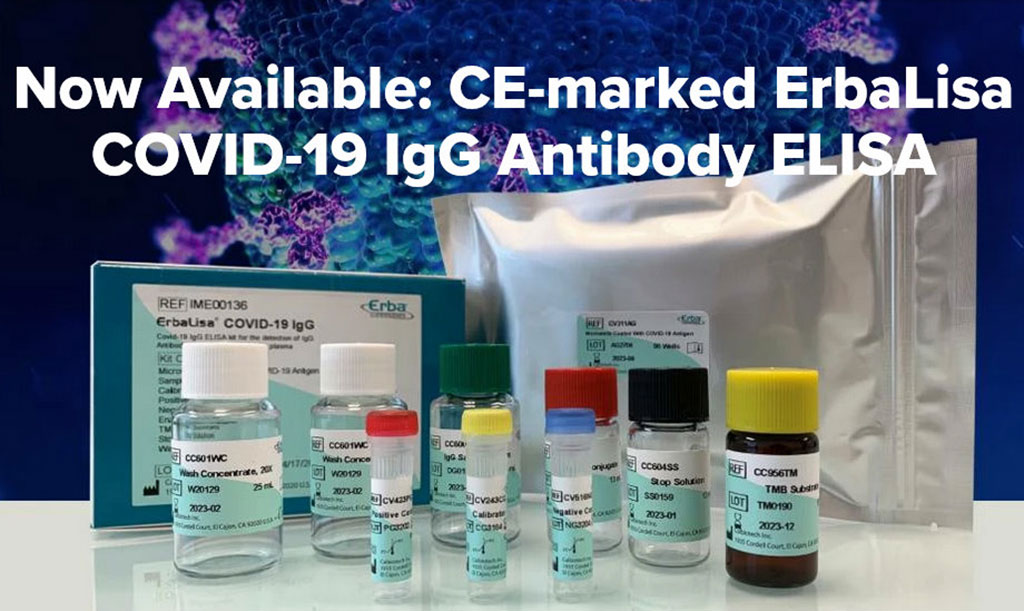
ERBA Mannheim Launches CE Marked COVID-19 Antibody ELISA Kits
Erba Mannheim (London, England) has launched its ErbaLisa COVID-19 ELISA kits for detection of IgG and IgM antibodies to SARS-CoV-2. The assays have been designed for use either manually or with any open automated ELISA analyzer, such as the Erba Mannheim ELAN 30s. More...29 Apr 2020

The IFCC Launches Online Resource for COVID-19 Diagnostics
The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), the umbrella organization encompassing national societies in the field in nearly 100 countries around the world, established an online resource providing key information on laboratory guidelines, biosafety, and other important matters to assist clinical laboratories as they face the challenges posed by the COVID-19 outbreak. More...28 Apr 2020
In Other News
Millions of 3D-Printed Nasopharyngeal Swabs for COVID-19 Testing to Reach Healthcare Providers
Leinco Technologies and Vanderbilt University Medical Center to Jointly Develop Rapid, POC, Immunodiagnostic COVID-19 Test
Millions of 3D-Printed Nasopharyngeal Swabs for COVID-19 Testing to Reach Healthcare Providers
Ortho’s Second COVID-19 Antibody Test with 100% Specificity Launched
Urea Dissociation Tests Reduces SARS-CoV-2 IgM False-Positives
Vela Diagnostics Receives CE-IVD Mark for COVID-19 Detection Test
CRISPR-Based Test Detects SARS-CoV-2 from Respiratory Swab RNA Extracts in 45 Minutes
SD Biosensor Coronavirus PCR Test Receives FDA Emergency Use Authorization
Enzo Biochem Launches Proprietary COVID-19 Diagnostic Test
Siemens to Offer Laboratory-Based SARS-CoV-2 Total Antibody Test with Time to First Result of 14 Minutes
ChromaCode Launches High-Throughput HDPCR SARS-CoV-2 Real-Time Assay
NovaBay to Distribute COVID-19 Antibody Rapid, Finger Prick Point-of-Care Test
Seegene's Allplex 2019-nCoV Assay Receives FDA Emergency Use Authorization
Molecular Beacons Technology Used in Rapid COVID-19 Tests
Rockefeller Foundation Lays Out Massive COVID-19 Testing Action Plan for US
Randox Unveils Whole Pathogen Molecular Controls for SARS-CoV-2 (COVID-19)
Vela Diagnostics Awarded BARDA Contract to Develop Manual and Automated COVID-19 Tests
GenoSensor COVID-19 RT-PCR KIT Granted FDA Emergency Use Authorization
ELITechGroup’s Rapid Detection Test Kits Secure FDA Emergency Use Authorization
Coronavirus-Detecting Breathing Device Could Potentially Give a Diagnosis in Less than One Minute
Accelerate Diagnostics Partners with BioCheck to Distribute CLIA Analyzer and SARS-CoV-2 Antibody Tests
Newly Launched Coronavirus Blood Test Identifies Past Exposure
Gold Standard Diagnostics Launches COVID-19 Antibody Tests











