CRISPR-Based Test Detects SARS-CoV-2 from Respiratory Swab RNA Extracts in 45 Minutes
By LabMedica International staff writers
Posted on 27 Apr 2020
A newly-published study of an assay for detecting SARS-CoV-2 from respiratory swab RNA extracts in less than 45 minutes contains the first peer-reviewed data using CRISPR diagnostics for COVID-19, with the largest set of patient samples to-date.Posted on 27 Apr 2020
In the study, Mammoth Biosciences (San Francisco, CA, USA) demonstrated how the diagnostic capabilities of CRISPR can be leveraged to offer a faster, lower-cost and visual alternative to traditional quantitative polymerase chain reaction (qRT-PCR) assays for diagnosing SARS-CoV-2. The researchers validated the method using contrived reference samples and clinical samples from US patients, including 36 patients with COVID-19 infection and 42 patients with other viral respiratory infections.
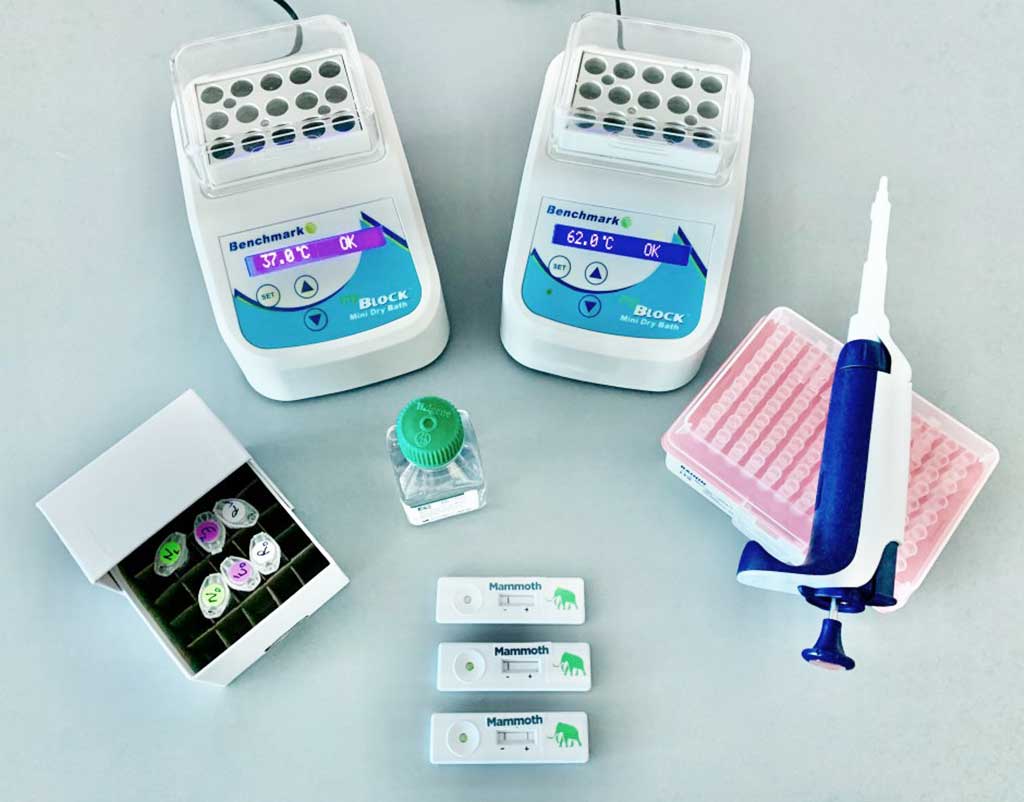
Image: DETECTR™ (Photo courtesy of Mammoth Biosciences)
The company’s CRISPR-based diagnostic assay, DETECTR, can deliver results in under 45 minutes as visualized on a lateral flow strip, similar to an at-home pregnancy test. DETECTR does not require a complex laboratory setting; it can be performed with portable heat blocks and readily available, “off-the-shelf” reagents and disposable lateral flow strips. The assay offers similar levels of sensitivity and specificity to qRT-PCR tests, with 95% positive predictive agreement and 100% negative predictive agreement.
“We need faster, more accessible and scalable diagnostics. The point-of-care testing space is ripe for disruption and CRISPR diagnostics have the potential to bring reliable testing to the most vulnerable environments,” said Mammoth’s Chief Technology Officer Janice Chen. “Because CRISPR can be programmed to detect any DNA or RNA sequence, we have been able to reconfigure our DETECTR platform within days to detect the SARS-CoV-2 virus from one of the first confirmed cases in the US, made possible by our collaboration with Dr. Charles Chiu at UCSF.”
Related Links:
Mammoth Biosciences














