
25-Second COVID-19 Saliva Test Delivers Real-Time Results with 98% Sensitivity and 100% Specificity
A non-invasive ultra-rapid COVID-19 saliva test delivers 98% sensitivity and 100% specificity in symptomatic and asymptomatic individuals with SARS-CoV-2, including variants, making it ideal for point-of-care (POC) diagnosis and rapid mass-screening. More...16 Mar 2021
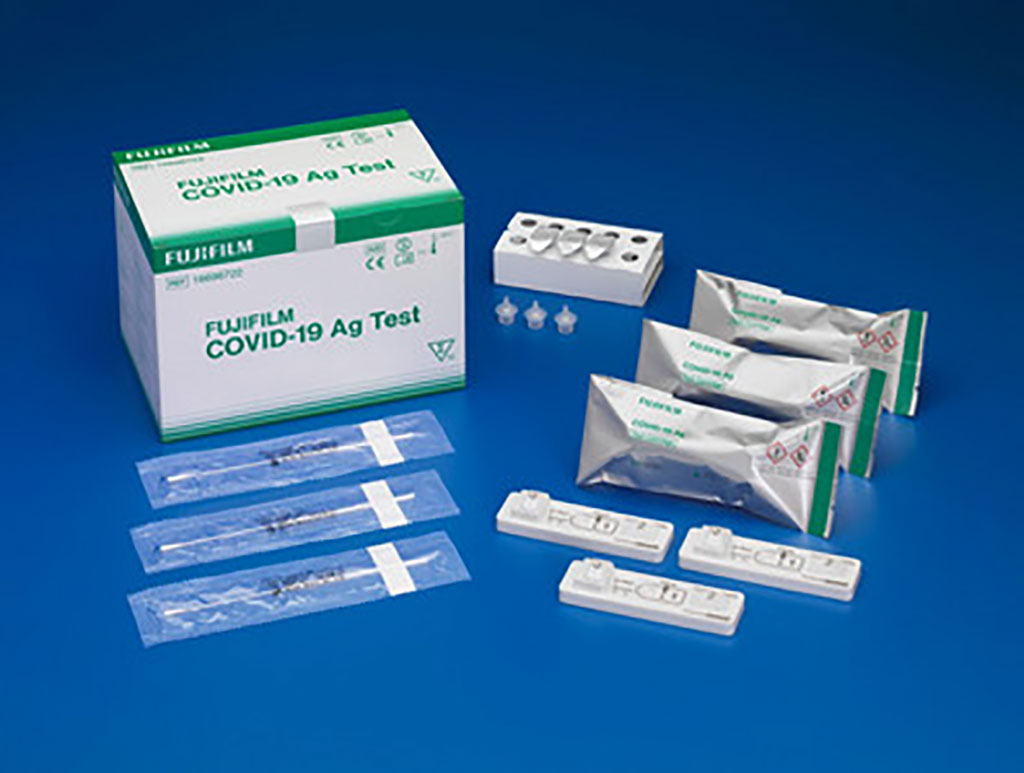
Fujifilm Launches Highly-Sensitive, Rapid CE-Marked SARS-CoV-2 Antigen Test Based on Silver Halide Amplification Technology
FUJIFILM Europe GmbH (Duesseldorf, Germany) has launched a new antigen test kit for the diagnosis of SARS-CoV-2 infection that has received the CE certificate and utilizes its proprietary silver amplification immunochromatography method. More...16 Mar 2021

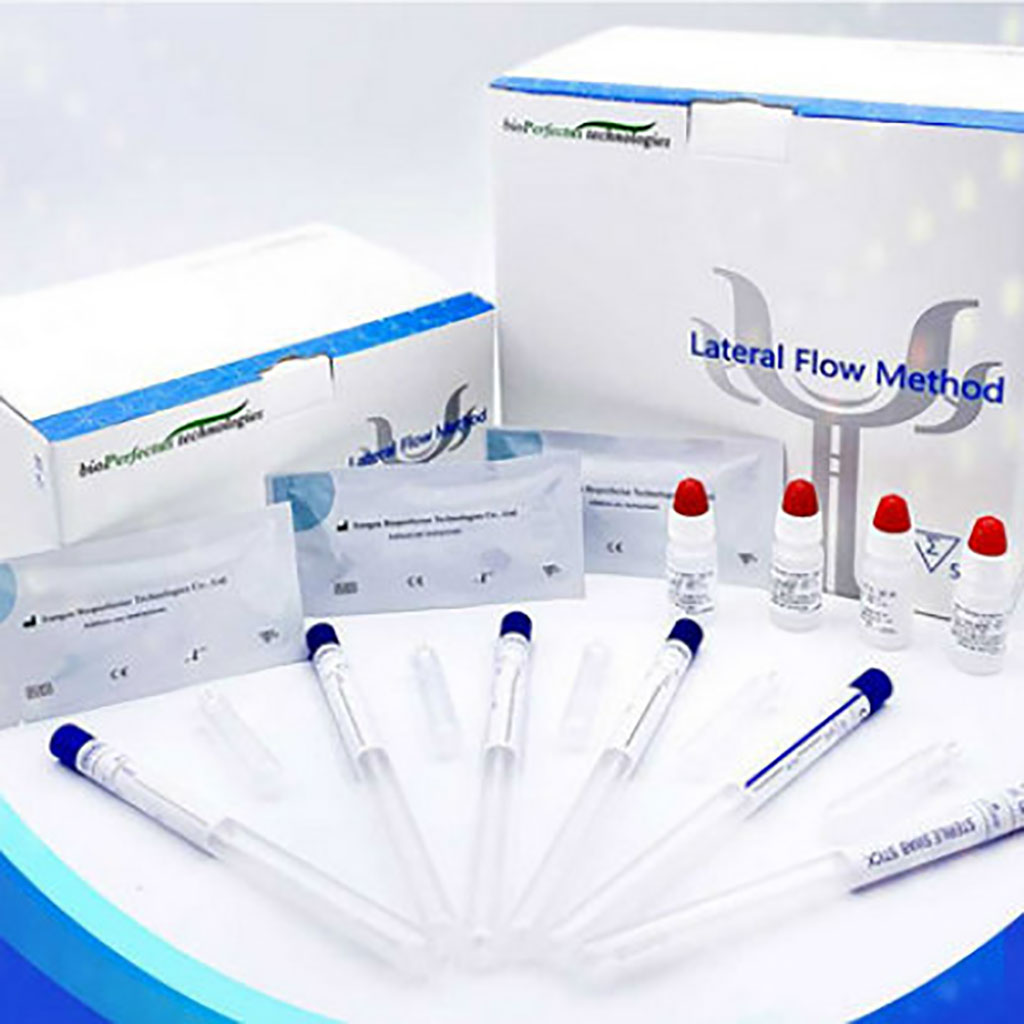
Bioperfectus SARS-CoV-2 Antigen Rapid Test Kit with High Sensitivity and Specificity Assists in Fight against COVID-19 Pandemic
Jiangsu Bioperfectus Technologies Co., Ltd.’s (Shanghai, China) Novel Corona Virus (SARS-CoV-2) Antigen Rapid Test Kit with clinically proven high sensitivity and specificity is playing an important role in COVID-19 diagnostics as a fast and cost-efficient antigen test. More...12 Mar 2021

New Assay Detects Brazilian and South African SARS-CoV-2 Variants Faster than Sequencing, Screens More Samples
SeqOnce Biosciences (Pasadena, CA, USA) has launched its new AzureSeq One-Step Universal RT-qPCR kit for the detection of the novel coronavirus SARS-CoV-2 E484K variant at a faster rate than sequencing with the ability to screen more samples. More...12 Mar 2021
First-Ever T Cell-Based Test to Detect Prior SARS-CoV-2 Infection Receives FDA Emergency Use Authorization
The first and only clinical T cell-based test for patients to detect the unique T-cell signature specific to SARS-CoV-2 has been granted Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA). More...12 Mar 2021
Thermo Fisher’s New Customizable TaqMan SARS-CoV-2 Mutation Panel Allows Labs to Choose Which Variants to Track
Thermo Fisher Scientific Inc. (Waltham, MA, USA) has launched its Applied Biosystems TaqMan SARS-CoV-2 Mutation Panel, a customizable menu of 22 verified real-time PCR assays for identification of SARS-CoV-2 mutations. More...11 Mar 2021
In Other News
New Fast and Easy-to-Perform COVID-19 Test Detects New Emerging SARS-CoV-2 Variants in Just Over One Hour
PerkinElmer Launches Comprehensive Solutions for Detecting SARS-CoV-2 Mutations
Pioneering COVID-19 Testing Platform Combines Saliva-Based Sampling, Pooled Testing and PCR Diagnostics
First-Ever COVID-19 Test Measures Neutralizing Antibodies that Prevent SARS-CoV-2 from Entering Host Cells
New Quantitative Strategy for Pooling COVID-19 Tests Could Detect Outbreaks Early
New COVID-19 Test Detects Cell Mediated (T Cell) Immune Response to SARS-CoV-2 Infection
Low-Cost and Fast COVID-19 Test Detects SARS-CoV-2 from Pool of Gargle Lavage Samples in One Hour
Rapid Molecular Diagnostic Platform Sends Precise COVID-19 RNA Results to Smartphone in Minutes
Seegene's Latest COVID-19 Test Simultaneously Targets Four SARS-CoV-2 Genes and Recognizes Multiple Virus Variants
Robotic Next Generation Sequencing Platform Accurately Screens Thousands of COVID-19 Samples at Once
Seegene Submits Allplex SARS-CoV-2/FluA/FluB/RSV Assay for US FDA Emergency Use Authorization
YHLO Launches New SARS-CoV-2 Neutralization Antibody (NAb) Assay
Next-Generation PCR-Based COVID-19 Test Can Distinguish SARS-CoV-2 Strains and Quantify Viral Load
Coronavirus-Like Nanoparticles Could Ensure Reliability of Simpler, Faster COVID-19 Tests
Rapid Lateral Flow Assays Detect COVID-19 Variants and Differentiate COVID-19 from Other Respiratory Viral Diseases
Diasys Launches New SARS-CoV-2 UTAB FS Universally Applicable Total Antibody Test
Thermo Fisher Launches SpeciMax Raw Saliva Collection Kit for SARS-CoV-2 Surveillance Testing
New Cloning Technique Can Accurately Assess Transmissibility of Future SARS-CoV-2 Mutants
First-Ever Clinical T-Cell Based Test for Patients Confirms Recent or Prior COVID-19 Infection
New Approach to Pooled COVID-19 Testing Could Be Highly Effective in Curbing SARS-CoV-2 Pandemic
Stamp-Sized Microfluidic Chip Uses Programmed Magnetic Nanobeads and Off-the-Shelf Cellphone to Diagnose COVID-19
Rapid Point-of-Care COVID-19 Antigen Test Based on Waveguide Technology Could Deliver Results in Under Six Minutes
New COVID-19 Test to Scan Saliva or Nasal Swabs Using Specialized Probes for Detecting SARS-CoV-2 RNA Sequences











