Liquid Biopsy Could Identify Advanced Breast Cancer Patients
|
By LabMedica International staff writers Posted on 09 Aug 2017 |

Image: The Ion Ampliseq library preparation kits (Photo courtesy of Thermo Fisher Scientific).
A novel blood test has been developed that measures genetic changes in circulating cancer DNA that could help identify patients with metastatic breast cancer who could benefit from a change of treatment.
Somatic mutation profiling of breast tumor tissues has identified a number of distinct breast cancer molecular subtypes characterized by diverse somatic mutations, including single nucleotide variants (SNVs) and copy number alterations (CNAs).
Scientists at the University of Leicester (Leicester, UK) recruited 42 patients with radiological-confirmed metastatic breast cancer (MBC) and nine women attending for breast screening mammography as age-matched controls. Blood samples were collected and the plasma processed using the Circulating Nucleic Acids kit.
The team designed a custom 158-amplicon panel (size range 125–175 bp) across 16 genes based on previous studies and publically available databases. Library preparation and Personal Genome Machine (PGM) sequencing were performed using the Ion Ampliseq library preparation kit. Droplet digital polymerase chain reaction (ddPCR) was used to validate tumor protein p53 (TP53), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and estrogen receptor 1 (ESR1) mutations.
The scientists identified no mutations in cell free DNA (cfDNA) of healthy controls, whereas exactly half the patients with metastatic breast cancer had at least one mutation or amplification in cfDNA (mean 2, range 1–6) across a total of 13 genes. Longitudinal follow up showed dynamic changes to mutations and gene amplification in cfDNA indicating clonal and subclonal response to treatment that was more dynamic than cancer antigen 15-3 (CA15-3).
At the time of blood sampling disease progression was occurring in seven patients with erb-b2 receptor tyrosine kinase 2 (ERBB2) gene amplification in their cfDNA and three of these patients were human epidermal growth factor receptor 2 (HER2) negative at diagnosis, suggesting clonal evolution to a more aggressive phenotype. Six of the women with hormone-driven cancers had mutations in the ESR1 gene, which has been linked to resistance to anti-hormone treatments.
David Guttery, PhD, a leading author of the study, said, “We have developed a novel blood test that can simultaneously detect somatic mutations and copy number alterations that are integral in driving the growth of breast cancer. By analyzing blood plasma to measure for cancer-specific changes to key breast cancer genes, including the HER2 and estrogen receptor genes, we hope this test could help doctors and patients choose the best treatment at the best time.” The study was described by Professor Jacqui A. Shaw, PhD, in an oral presentation at the Frank May Prize Lecture on June 26, 2017, at the University of Leicester.
Related Links:
University of Leicester
Somatic mutation profiling of breast tumor tissues has identified a number of distinct breast cancer molecular subtypes characterized by diverse somatic mutations, including single nucleotide variants (SNVs) and copy number alterations (CNAs).
Scientists at the University of Leicester (Leicester, UK) recruited 42 patients with radiological-confirmed metastatic breast cancer (MBC) and nine women attending for breast screening mammography as age-matched controls. Blood samples were collected and the plasma processed using the Circulating Nucleic Acids kit.
The team designed a custom 158-amplicon panel (size range 125–175 bp) across 16 genes based on previous studies and publically available databases. Library preparation and Personal Genome Machine (PGM) sequencing were performed using the Ion Ampliseq library preparation kit. Droplet digital polymerase chain reaction (ddPCR) was used to validate tumor protein p53 (TP53), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and estrogen receptor 1 (ESR1) mutations.
The scientists identified no mutations in cell free DNA (cfDNA) of healthy controls, whereas exactly half the patients with metastatic breast cancer had at least one mutation or amplification in cfDNA (mean 2, range 1–6) across a total of 13 genes. Longitudinal follow up showed dynamic changes to mutations and gene amplification in cfDNA indicating clonal and subclonal response to treatment that was more dynamic than cancer antigen 15-3 (CA15-3).
At the time of blood sampling disease progression was occurring in seven patients with erb-b2 receptor tyrosine kinase 2 (ERBB2) gene amplification in their cfDNA and three of these patients were human epidermal growth factor receptor 2 (HER2) negative at diagnosis, suggesting clonal evolution to a more aggressive phenotype. Six of the women with hormone-driven cancers had mutations in the ESR1 gene, which has been linked to resistance to anti-hormone treatments.
David Guttery, PhD, a leading author of the study, said, “We have developed a novel blood test that can simultaneously detect somatic mutations and copy number alterations that are integral in driving the growth of breast cancer. By analyzing blood plasma to measure for cancer-specific changes to key breast cancer genes, including the HER2 and estrogen receptor genes, we hope this test could help doctors and patients choose the best treatment at the best time.” The study was described by Professor Jacqui A. Shaw, PhD, in an oral presentation at the Frank May Prize Lecture on June 26, 2017, at the University of Leicester.
Related Links:
University of Leicester
Latest Pathology News
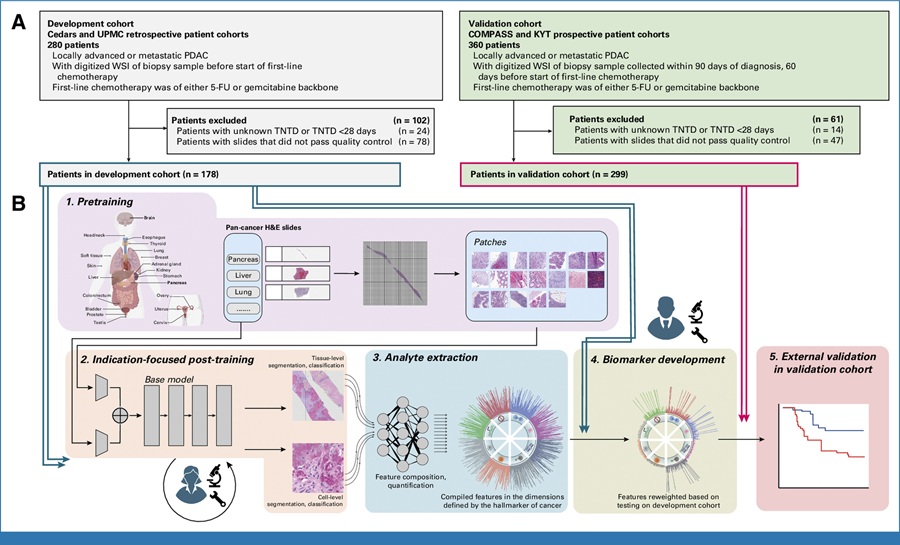
- AI Tool Predicts Chemotherapy Response from Biopsy Slides
- Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
- World’s First Optical Microneedle Device to Enable Blood-Sampling-Free Clinical Testing
- Novel mcPCR Technology to Transform Testing of Clinical Samples
- Pathogen-Agnostic Testing Reveals Hidden Respiratory Threats in Negative Samples
- Molecular Imaging to Reduce Need for Melanoma Biopsies
- Urine Specimen Collection System Improves Diagnostic Accuracy and Efficiency
- AI-Powered 3D Scanning System Speeds Cancer Screening
- Single Sample Classifier Predicts Cancer-Associated Fibroblast Subtypes in Patient Samples
- New AI-Driven Platform Standardizes Tuberculosis Smear Microscopy Workflow
- AI Tool Uses Blood Biomarkers to Predict Transplant Complications Before Symptoms Appear
- High-Resolution Cancer Virus Imaging Uncovers Potential Therapeutic Targets
- Research Consortium Harnesses AI and Spatial Biology to Advance Cancer Discovery
- AI Tool Helps See How Cells Work Together Inside Diseased Tissue
- AI-Powered Microscope Diagnoses Malaria in Blood Smears Within Minutes
- Engineered Yeast Cells Enable Rapid Testing of Cancer Immunotherapy
Channels
Clinical Chemistry
view channel
Blood Test Tracks Transplant Health Using Donor DNA
Organ transplantation offers life-saving treatment for patients with end-stage disease, but complications such as rejection remain a constant risk. Monitoring transplanted organs typically relies on invasive... Read more
AI Sensor Detects Neurological Disorders Using Single Saliva Drop
Neurological disorders such as Parkinson’s disease and Alzheimer’s disease often develop gradually and present subtle symptoms in their early stages. Because early signs are frequently vague or atypical,... Read moreHematology
view channel
Rapid Cartridge-Based Test Aims to Expand Access to Hemoglobin Disorder Diagnosis
Sickle cell disease and beta thalassemia are hemoglobin disorders that often require referral to specialized laboratories for definitive diagnosis, delaying results for patients and clinicians.... Read more
New Guidelines Aim to Improve AL Amyloidosis Diagnosis
Light chain (AL) amyloidosis is a rare, life-threatening bone marrow disorder in which abnormal amyloid proteins accumulate in organs. Approximately 3,260 people in the United States are diagnosed... Read moreImmunology
view channel
Cancer Mutation ‘Fingerprints’ to Improve Prediction of Immunotherapy Response
Cancer cells accumulate thousands of genetic mutations, but not all mutations affect tumors in the same way. Some make cancer cells more visible to the immune system, while others allow tumors to evade... Read more
Immune Signature Identified in Treatment-Resistant Myasthenia Gravis
Myasthenia gravis is a rare autoimmune disorder in which immune attack at the neuromuscular junction causes fluctuating weakness that can impair vision, movement, speech, swallowing, and breathing.... Read more
New Biomarker Predicts Chemotherapy Response in Triple-Negative Breast Cancer
Triple-negative breast cancer is an aggressive form of breast cancer in which patients often show widely varying responses to chemotherapy. Predicting who will benefit from treatment remains challenging,... Read moreBlood Test Identifies Lung Cancer Patients Who Can Benefit from Immunotherapy Drug
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options, and even newly approved immunotherapies do not benefit all patients. While immunotherapy can extend survival for some,... Read moreMicrobiology
view channel
Study Highlights Accuracy Gaps in Consumer Gut Microbiome Kits
Direct-to-consumer gut microbiome kits promise personalized insights by profiling fecal bacteria and generating health readouts, but their analytical accuracy remains uncertain. A new study shows that... Read more
WHO Recommends Near POC Tests, Tongue Swabs and Sputum Pooling for TB Diagnosis
Tuberculosis (TB) remains one of the world’s leading infectious disease killers, yet millions of cases go undiagnosed or are detected too late. Barriers such as reliance on sputum samples, limited laboratory... Read morePathology
view channel
Novel mcPCR Technology to Transform Testing of Clinical Samples
DNA methylation is an important biological marker used in the diagnosis and monitoring of many diseases, including cancer. These chemical modifications to DNA influence gene activity and can reveal early... Read more
Sex Differences in Alzheimer’s Biomarkers Linked to Faster Cognitive Decline
Sex differences in Alzheimer’s disease present ongoing diagnostic challenges, with women often experiencing a disproportionate disease burden even when preclinical amyloid-beta levels are similar to men.... Read moreTechnology
view channel
AI Model Outperforms Clinicians in Rare Disease Detection
Rare diseases affect an estimated 300 million people worldwide, yet diagnosis is often protracted and error-prone. Many conditions present with heterogeneous signs that overlap with common disorders, leading... Read more
AI-Driven Diagnostic Demonstrates High Accuracy in Detecting Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) is a rare but serious complication affecting 1% to 2% of primary joint replacement surgeries. The condition occurs when bacteria or fungi infect tissues around an implanted... Read moreIndustry
view channel
Agilent Technologies Acquires Pathology Diagnostics Company Biocare Medical
Agilent Technologies (Santa Clara, CA, USA) has entered into a definitive agreement to acquire Biocare Medical (Pacheco, CA, USA), expanding its pathology portfolio through the addition of highly complementary... Read more
Cepheid Joins CDC Initiative to Strengthen U.S. Pandemic Testing Preparednesss
Cepheid (Sunnyvale, CA, USA) has been selected by the U.S. Centers for Disease Control and Prevention (CDC) as one of four national collaborators in a federal initiative to speed rapid diagnostic technologies... Read more
QuidelOrtho Collaborates with Lifotronic to Expand Global Immunoassay Portfolio
QuidelOrtho (San Diego, CA, USA) has entered a long-term strategic supply agreement with Lifotronic Technology (Shenzhen, China) to expand its global immunoassay portfolio and accelerate customer access... Read more








 (3) (1).png)