Fluorescent Reagents Evaluated for Vulvovaginal Candidiasis Diagnosis
By LabMedica International staff writers
Posted on 29 Jul 2021
Vulvovaginal candidiasis (VVC) is a common vaginitis in females, which is estimated to be the second most common cause of vaginitis after bacterial vaginosis. Vulvovaginal candidiasis affects around 70%–75% of all women during their childbearing age, and is considered to be an important public health problem.Posted on 29 Jul 2021
The morbidity associated with VVC makes it a major cause of causing pain, great discomfort, mental distress, anxiety, altered self-esteem, impairing work performance and interfering with sexual and affective relations. The signs of VVC are typically characterized white clumpy discharge. The commonly used diagnostic method is 10% potassium hydroxide (KOH) smear microscopy.
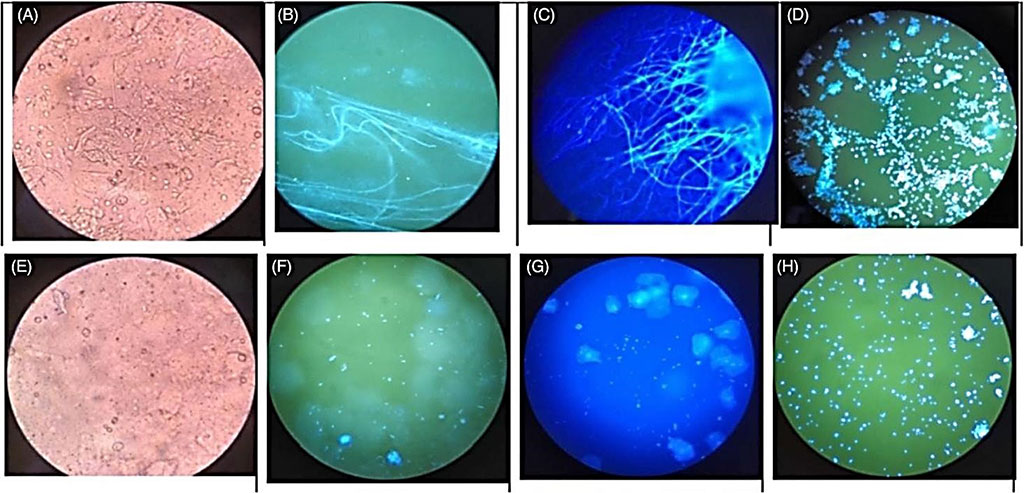
Image: The images under fluorescence microscopy and light microscopy with four methods. A-D, show Candida albicans, and E-H, show C. glabrata; both the fungal color and the structure in FB 85 and CFW were clear and easily recognized from the background squamous cells compared with the KOH method (Photo courtesy of Capital Medical University)
Clinical Laboratorians at the Capital Medical University (Beijing, China) and their associates recruited patients according to the symptoms and the signs included vaginal discharge, irritation, itching, and/or odor, and who were suspected of having VVC, from October 2018 to February 2019. A total of 110 specimens were obtained with suspected VVC, aged 19–60 years, with an average age of 30.99 ± 7.77 years.
The team used different methods to detect VVC: fungal culture, fluorescent microscopy observation with Calcofluor White (CFW) and fluorescent brightener 85 (FB 85). The swabs were smeared on two separate slides, one with a drop of CFW, the other with a drop of 0.1% FB 85. The secretions gathered from the dilator were smeared on a third slide and examined using KOH under a light microscopy by adding a drop of 10% KOH to the slide. Microscopic observations were conducted using an Olympus CX23 LEDRFSIC microscopy (Olympus Co., Ltd, Tokyo, Japan) using either the 340–380 nm ultraviolet light mode or the visible light mode.
The scientists reported that in the 110 specimens, 75/110 (68.2%) were positive by the KOH method, 71/110 (64.5%) were positive by the CFW fluorescent method, 68/110 (61.8%) were positive by the FB 85 fluorescent method, and 77/110 (77%) were positive by the fungal culture method. In the 110 specimens, the specificity of the three methods was 100%. The sensitivity, and accuracy of CFW were the highest, followed by KOH method and FB 85 was the lowest.
For the VVC caused by Candida glabrata, yeast form was always observed, which is very clear with the CFW method and the FB 85 method, but was not easily found by light microscopy. The team observed a fade time of two fluorescent reagents under daylight in the room environment and due to the crystals and the faint fluorescence, it was harder to distinguish the fungi from the 24 hours specimen than the fresh specimen.
The authors concluded that they could find pathogenic fungi more quickly with fluorescent method in diagnosis of VVC. Both CFW and FB 85 were good reagents. FB 85 is a new fluorescent reagent for the diagnosis of VVC and other fungal affection diseases and has potential value for clinical applications. The study was published on July 12, 2021 in the Journal of Clinical Laboratory Analysis.
Related Links:
Capital Medical University
Olympus Co., Ltd














