Blood-Based Mutation Testing Receives Certification
By LabMedica International staff writers
Posted on 18 Jun 2013
A blood-based mutation test has received Clinical Laboratory Improvement Amendments (CLIA) licensure for use in a commercial clinical laboratory. Posted on 18 Jun 2013
The blood test is based on BEAMing (Beads, Emulsion, Amplification, and Magnetics) technology which combines emulsion based digital polymerase chain reaction (PCR) with flow cytometry.
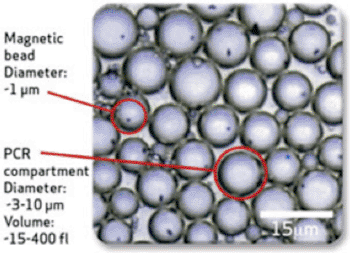
Image: BEAMing technology (Photo courtesy of Inostics).
The OncoBEAM (Inostics; Baltimore, MD, USA) blood tests enable the highly sensitive analysis of circulating tumor-specific DNA shed in the blood stream from primary and metastatic tumors. Due to its repeatability and noninvasiveness, the OncoBEAM tests introduce new possibilities for the management of cancers patients in the context of therapeutic selection and stratification, real time follow-up and resistance monitoring.
BEAMing technology converts single DNA molecules into single magnetic beads, each bead possessing thousands of copies of the original DNA molecule. The proportion of one kind of DNA molecule in a population of DNA molecules can be assessed by fluorescent staining and counting via flow cytometry. Flow sorting allows the separation of specific variants that can be verified for further study.
Due to its noninvasiveness, OncoBEAM blood tests introduce new possibilities for the management of various cancers like skin, colorectal, breast, and lung cancer. Now a simple blood draw can support clinical decision making in the context of therapy selection, assessment of drug response, resistance and recurrence monitoring, and detection of minimal residual disease.
Frank Diehl, PhD, the chief scientific officer of Inostics, said, “Due to the noninvasiveness and real-time reflection of tumor genetics, the OncoBEAM blood test represents a valuable tool to complement clinical decision making. We are extremely excited to be able to bring this solution to cancer patients as OncoBEAM tests offer a significant enhancement to clinical care over traditional tissue-based molecular testing."
Related Links:
Inostics