New Raman Microscopy Technique Achieves High-Resolution Images of Biological Samples
Posted on 31 Dec 2024
Understanding the behavior of the molecules and cells that make up our bodies is essential for advancing medical science. This has driven ongoing efforts to obtain clear images of biological processes beyond what the human eye can observe. Raman microscopy is an effective imaging technique for biological samples, as it can provide chemical insights into specific molecules, such as proteins, involved in bodily functions. However, the Raman light emitted by biological samples is weak, often leading to the signal being drowned out by background noise, which results in low-quality images. Researchers have employed cryogenic freezing in a new study to enhance the resolution of Raman microscopy images of biological samples.
The study published in Science Advances by researchers at Osaka University (Osaka, Japan) presents a method for achieving high-resolution Raman microscopy images. The team developed a microscope capable of maintaining the temperature of samples that had been frozen before imaging. This technique allowed the researchers to produce images that are up to eight times brighter than those previously obtained with Raman microscopy. Importantly, this method does not require staining or chemicals to fix the cells, enabling a more authentic view of cellular processes and behavior.
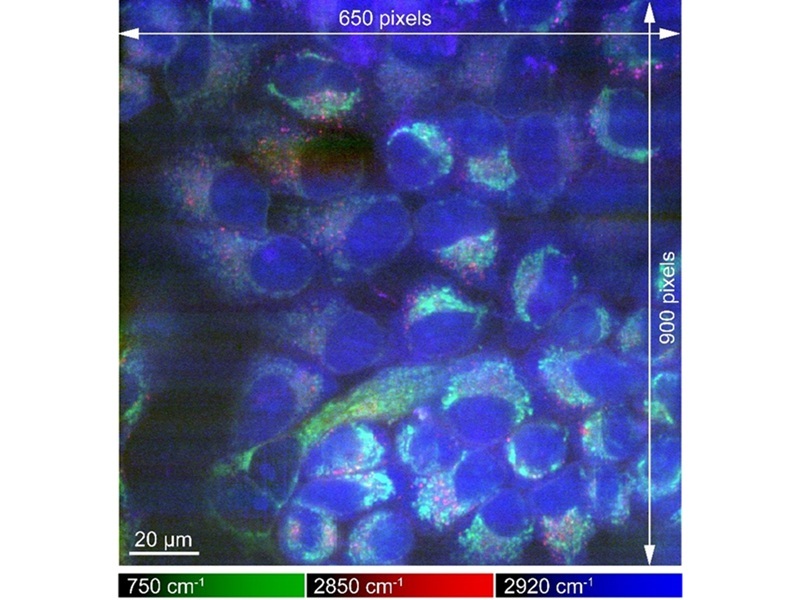
Furthermore, the researchers confirmed that the freezing process preserved the physicochemical states of various proteins, providing a key advantage over traditional chemical fixation methods. The new cryofixing technique can be integrated with other microscopy approaches, allowing for more detailed analysis of biological samples. It holds significant potential for a broad range of applications in the biological sciences, including medicine and pharmaceuticals.
"One of the main reasons for blurry images is the motion of the things you’re trying to look at," said Kenta Mizushima, lead author of the study. "By imaging frozen samples that were unable to move, we could use longer exposure times without damaging the samples. This led to high signals compared with the background, high resolution, and larger fields of view."













