New Reagent Aids in Diagnosis of Connective Tissue Disease in Hard-to-Detect Autoimmune Conditions
Posted on 19 Oct 2023
The increasing incidence of autoimmune diseases like rheumatoid arthritis, lupus, and multiple sclerosis has created great demand for accurate and reliable diagnostic methods. As patients become more knowledgeable about the symptoms and risks of these diseases, they are seeking quicker testing solutions for early detection and management. Reagents and consumables are a key component in the diagnostic process of autoimmune disorders. Now, a new reagent can aid in the diagnosis of connective tissue disease in hard-to-diagnose autoimmune diseases, thus speeding up diagnosis and enhancing patient outcomes.
Werfen (Barcelona, Spain) has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Aptiva Connective Tissue Disease (CTD) Essential reagent. This product complements the company's already FDA-approved Aptiva Celiac Disease reagent. Apart from CTD and Celiac Disease tests, Aptiva is working on diagnostics for other autoimmune conditions and has more than 60 analytes at different stages of advanced development. These analytes have the capability to shorten the diagnostic time for autoimmune diseases and contribute to more effective patient care.
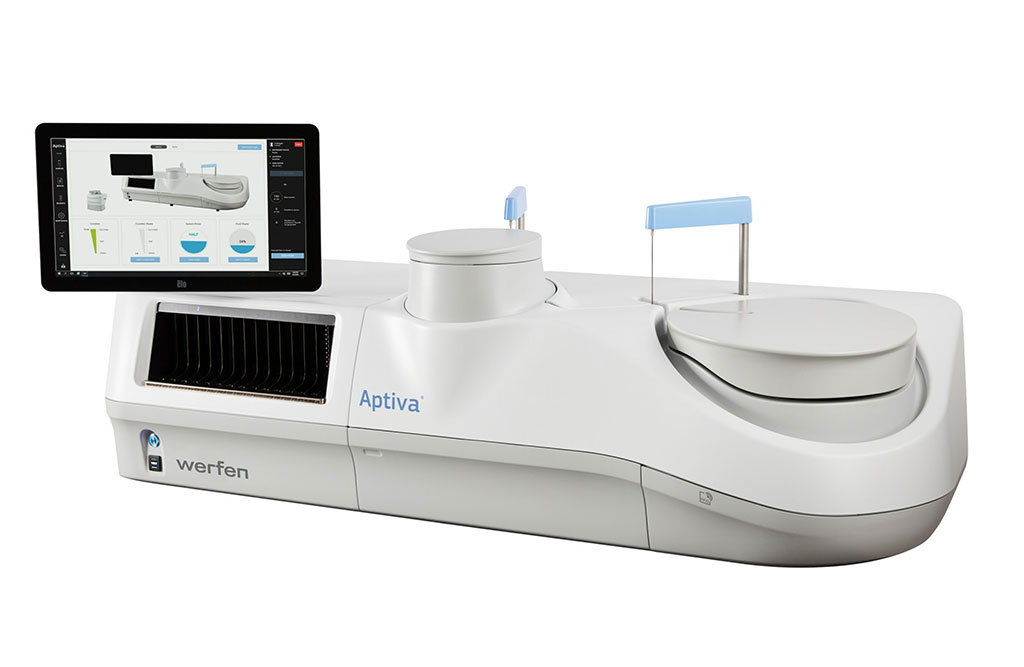
The Aptiva system is a fully automated multi-analyte platform that marks the next generation of high-throughput multi-analyte systems for clinical autoimmune labs. This system employs particle-based multi-analyte technology (PMAT) which simultaneously processes multiple analytes using a single patient sample. Thanks to PMAT, the Aptiva CTD Essential reagent can yield of up to 600 results per hour and enable labs to handle their testing volumes with minimal hands-on time.
"Aptiva CTD Essential with its unique biomarker composition and high level of analytical and clinical performance represents a breakthrough that fundamentally enhances the utility of diagnostic testing in the autoimmune laboratory," said Michael Mahler, PhD, Vice President of Research and Business Development at Werfen. "We are excited to bring this latest expansion of the Aptiva reagent menu to the US market, resulting in advanced patient care."
Related Links:
Werfen














