Simple Test Could Reduce Treatment of Bladder Cancer
By LabMedica International staff writers
Posted on 10 Jul 2018
Bladder cancer is relatively common and imposes the highest per patient cost on the healthcare system of the USA than the management of any other cancer type. Most bladder cancers are early-stage tumors known as papillary non-muscle-invasive bladder cancer (NMIBC).Posted on 10 Jul 2018
The decision whether to treat bladder cancer aggressively has been difficult as predictive diagnostic data is limited. Up to 70% of patients treated for early stage lesions that have not invaded the bladder wall will experience recurrence of these lesions, and 20% of these patients will develop an invasive cancer.
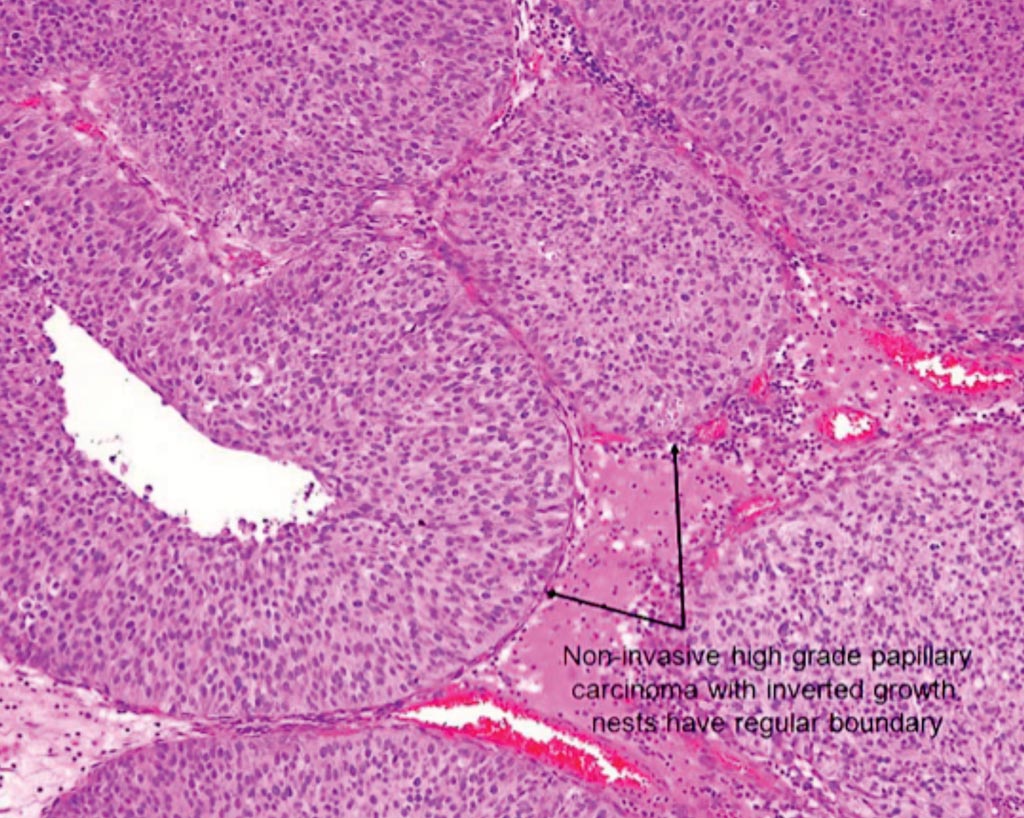
Image: A histopathology of papillary non–muscle-invasive bladder cancer (Photo courtesy of American Urological Association).
Scientists from the Georgetown University Medical Center (Washington, DC, USA) and their Danish counterparts initially evaluated in a cohort of 82 patients with papillary NMIBC (“Georgetown cohort”). Next, the value of Stromal Antigen 2 gene (STAG2) immunostaining for prediction of progression to muscle invasion was evaluated in a progressor-enriched cohort of 253 patients with papillary NMIBC (“Aarhus cohort”). The test involves examining bladder tumors that had been removed during initial surgery for over expression of the STAG2 gene.
In the Georgetown cohort, 52% of NMIBC tumors with intact STAG2 expression recurred, whereas 25% of STAG2-deficient tumors recurred. Multivariable analysis identified intact STAG2 expression as an independent predictor of recurrence. In the progressor-enriched Aarhus cohort, 38% of tumors with intact STAG2 expression progressed within five years, versus 16% of STAG2-deficient tumors. Multivariable analysis identified intact STAG2 expression as an independent predictor of progression. The authors stated that the test could, in some cases, spare patients constant surveillance and, in others, support forgoing aggressive treatment that can produce significant side effects.
Todd Waldman, MD, PhD, a professor of oncology and senior author of the study said, “We are closer to our goal of lowering the risk of both aggressive bladder cancer and over-surveillance and treatment side effects in bladder cancer patients. In principle, it might be possible to reduce the frequency of post-resection surveillance and therapy in patients whose cancer is STAG2-negative, and, conversely, treat patients and keep up high frequency surveillance in patients who have positive test results.” The study was published on June 28, 2018, in the journal Clinical Cancer Research.
Related Links:
Georgetown University Medical Center













