Blood Test May Predict Recurrence of Breast Cancer
By LabMedica International staff writers
Posted on 20 Dec 2017
The use of measuring circulating tumor cells (CTC) blood test in a new way has been explored. Currently, the test is FDA-approved for use by physicians to monitor response to treatment in patients with advanced breast, colon or prostate cancer, but not early stage cancer.Posted on 20 Dec 2017
A rise in the number of circulating tumor cells in the blood in patients with advanced disease may indicate trouble before it shows up on a scan. This concept has been evaluated as test in a different setting, with individuals alive and cancer-free about five years after their diagnosis and potentially cured, but still at risk for having a recurrence of their disease.
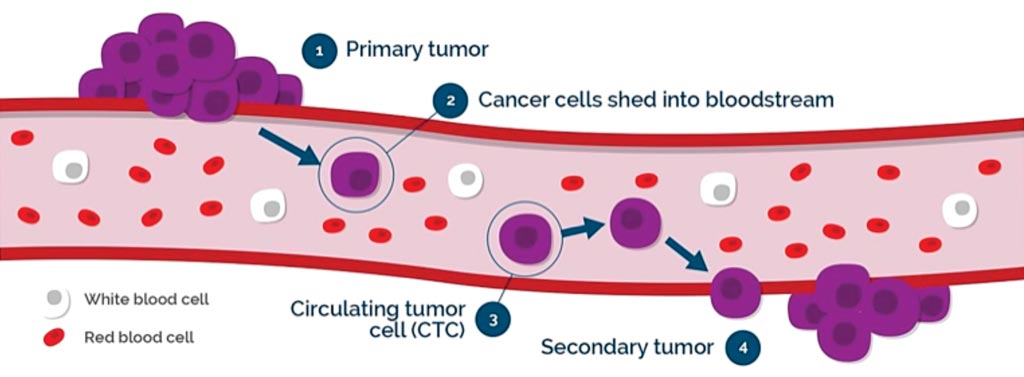
Image: A diagram of circulating tumor cells in the bloodstream (Photo courtesy of Vortex Biosciences).
Scientists working with the ECOG-ACRIN Cancer Research Group (Philadelphia, PA, USA) performed the test on a single blood sample provided by 547 breast cancer patients who had been diagnosed more than five years prior and treated as part of a large ECOG-ACRIN breast cancer treatment trial, E5103. This group of patients had stage two or three breast cancer, and the cells in their tumors were human epidermal growth factor receptor 2 (HER2)- negative. Many women in E5103 remain cancer free and are being followed for their breast cancer status as part of standard care.
Joseph A. Sparano, MD, vice chair of the ECOG-ACRIN Cancer Research Group, said, “Late recurrence five or more years after surgery accounts for at least one-half of recurrences of breast cancer, and there are no tests that identify who is at highest risk. We found that in women who were cancer-free five years after diagnosis, about 5% had a positive CTC test. More importantly we also found that a positive test was associated with a 35% recurrence risk after two years, compared with only 2% for those with a negative CTC test.”
Dr. Sparano added “Our ultimate goal is to use blood tests like this to tailor treatment in a way that minimizes recurrence risk for those at high risk, and spare treatment for those at low risk who may be unlikely to benefit from it. The findings of this analysis provide strong evidence to further evaluate this new risk assessment approach using CTC and other blood-based tests in this setting”. The study was presented at the 40th annual San Antonio Breast Cancer Symposium held December 5 - 9, 2017, in San Antonio, TX, USA.
Related Links:
ECOG-ACRIN Cancer Research Group













