New Molecular Test Identifies Colorectal Cancer
By LabMedica International staff writers
Posted on 23 Aug 2017
Colorectal cancer is one of the leading causes of cancer-related deaths worldwide, with more than 600,000 deaths each year. Among individuals undergoing treatment for the disease, recurrence occurs in 30% to 50% of all cases. The majority of these cases present in the first two to three years following initial diagnosis and treatment.Posted on 23 Aug 2017
A clinically validated blood test that is designed to help detect colorectal cancer (CRC) recurrence by measuring fragments of genetic material, circulating tumor DNA (ctDNA), that leak from a tumor into the blood stream. With this test it may be possible to identify CRC recurrence in advance of symptoms before other tests indicate recurrence.
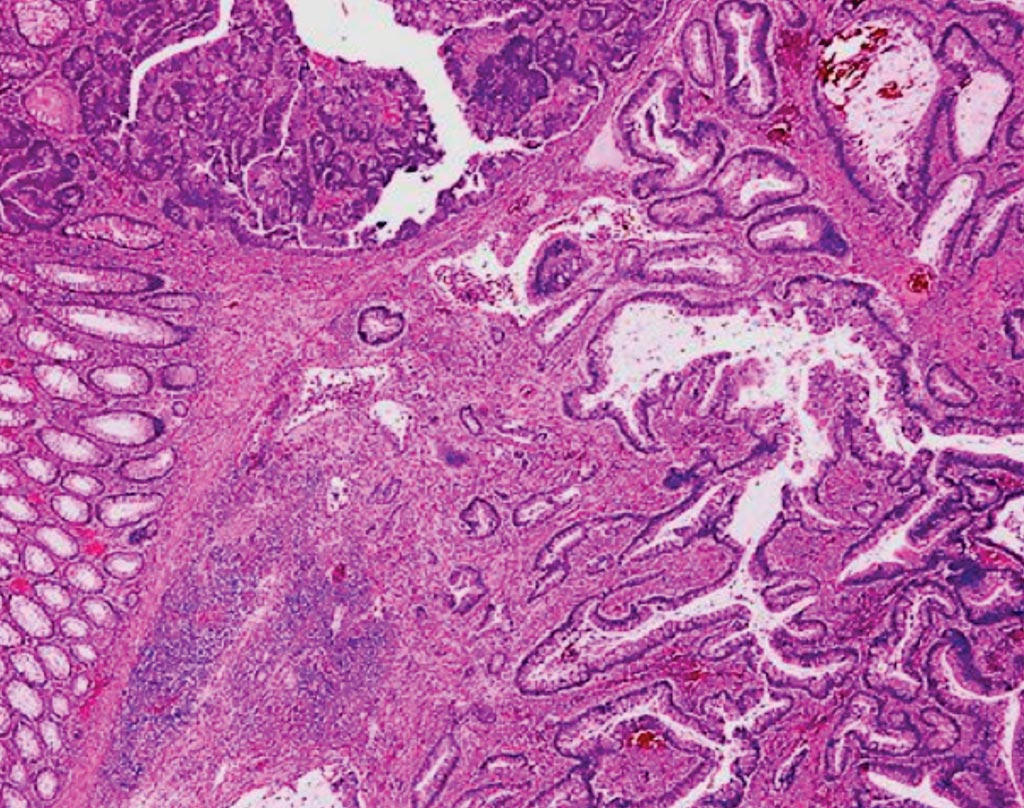
Image: A histologic section of a colonic adenoma containing invasive carcinoma (Photo courtesy of Dr. Mauro Risio).
In a previously published study, clinical data showed that the liquid biopsy test Colvera (Clinical Genomics, North Ryde, NSW, Australia) detected twice the number of recurrence cases as carcinoembryonic antigen (CEA) testing, the current guidelines-recommended blood test used for CRC recurrence monitoring. As Colvera only requires a peripheral blood sample, the test can be administered along with other CRC surveillance tests, including CEA.
Colvera is a polymerase chain reaction (PCR)-based test measuring two epigenetically modified genes, hyper-methylated Branched Chain Amino Acid Transaminase 1, (BCAT1) and IKAROS Family Zinc Finger (IKZF1), in free circulating DNA which may leak from cancerous lesions into the bloodstream. This product is the result of almost a decade of research, development and clinical validation studies, including testing in more than 4,000 volunteers. Colvera does not require specialized equipment or modifications to clinical protocol.
Lawrence LaPointe, PhD, President and CEO of Clinical Genomics, said, “Colorectal cancer outcomes improve with early detection but currently available monitoring tests frequently fail to detect disease at a point when clinical intervention can be effective. This failure is a key reason why mortality from colorectal cancer is so high. Colvera is a new test that can detect molecular changes in circulating tumor DNA associated with cancer development. Colvera provides physicians with actionable information that can trigger further clinical assessment. The overall aim is to improve survival through early detection.”
Related Links:
Colvera














