Cancer Biomarkers Identify Candidates for Aggressive Treatment
By LabMedica International staff writers
Posted on 26 Jan 2017
Prostate cancer (PC) is the second leading cause of male cancer death in the USA with an estimated 26,000 deaths in 2016. Two-thirds of all PC deaths observed in the US are men with localized disease who developed metastasis.Posted on 26 Jan 2017
Several biomarkers for dying from prostate cancer exist, but whether these are markers for telling who is likely to die early from any cause, and how their performance compares, is unknown. Identifying such a marker is important because physicians can then identify which men may benefit from new, more aggressive treatments for prostate cancer.
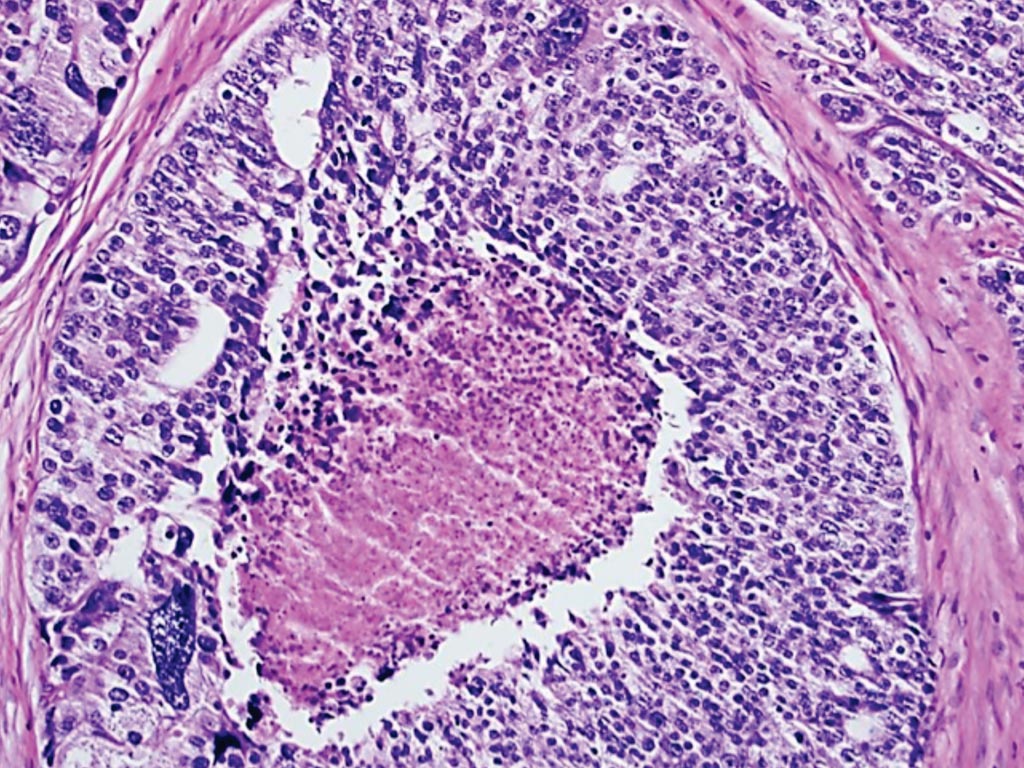
Image: A histopathology of prostate cancer – The presence of luminal comedo necrosis in a cancer gland space is sufficient to diagnose Gleason grade 5 (Photo courtesy of Kenneth Iczkowski, MD).
Oncologists at the Brigham and Women’s Hospital and their colleagues conducted a randomized clinical trial of 206 men with unfavorable-risk prostate cancer who were seen at a Harvard-affiliated academic hospital or an associated community hospital between December 1, 1995, to April 15, 2001.The men were identified, randomized to radiation therapy alone or radiation therapy followed by six months of androgen deprivation therapy, and followed for a median 16.62 years. A subgroup of 157 men with minimal comorbidities or no comorbidity were analyzed.
The team ascertained and compared the performance of four candidate surrogates (prostate-specific antigen [PSA] failure, PSA nadir greater than 0.5 ng/mL, PSA doubling time less than nine months, and interval to PSA failure (less than 30 months) for all-cause mortality using the proportion of treatment-effect metric. The investigators found that a prostate specific antigen (PSA) nadir (the lowest level a PSA drops after treatment) greater than 0.5 ng/mL following radiation and androgen deprivation therapy (anti-hormone therapy), appears to identify men prior to PSA failure who are at high-risk for dying early as a result of treatment failure for their prostate cancer.
Anthony Victor D'Amico, MD, PhD, chief of Genitourinary Radiation Oncology, and the senior author of the study, said “This study's results can have practice changing implications on how future prostate cancer trials are designed in terms of identifying the men for these studies who are at high risk for early death due to ineffective initial treatment for their prostate cancer.” The study was published on January 12, 2017, in the journal JAMA Oncology.













